Signalment:
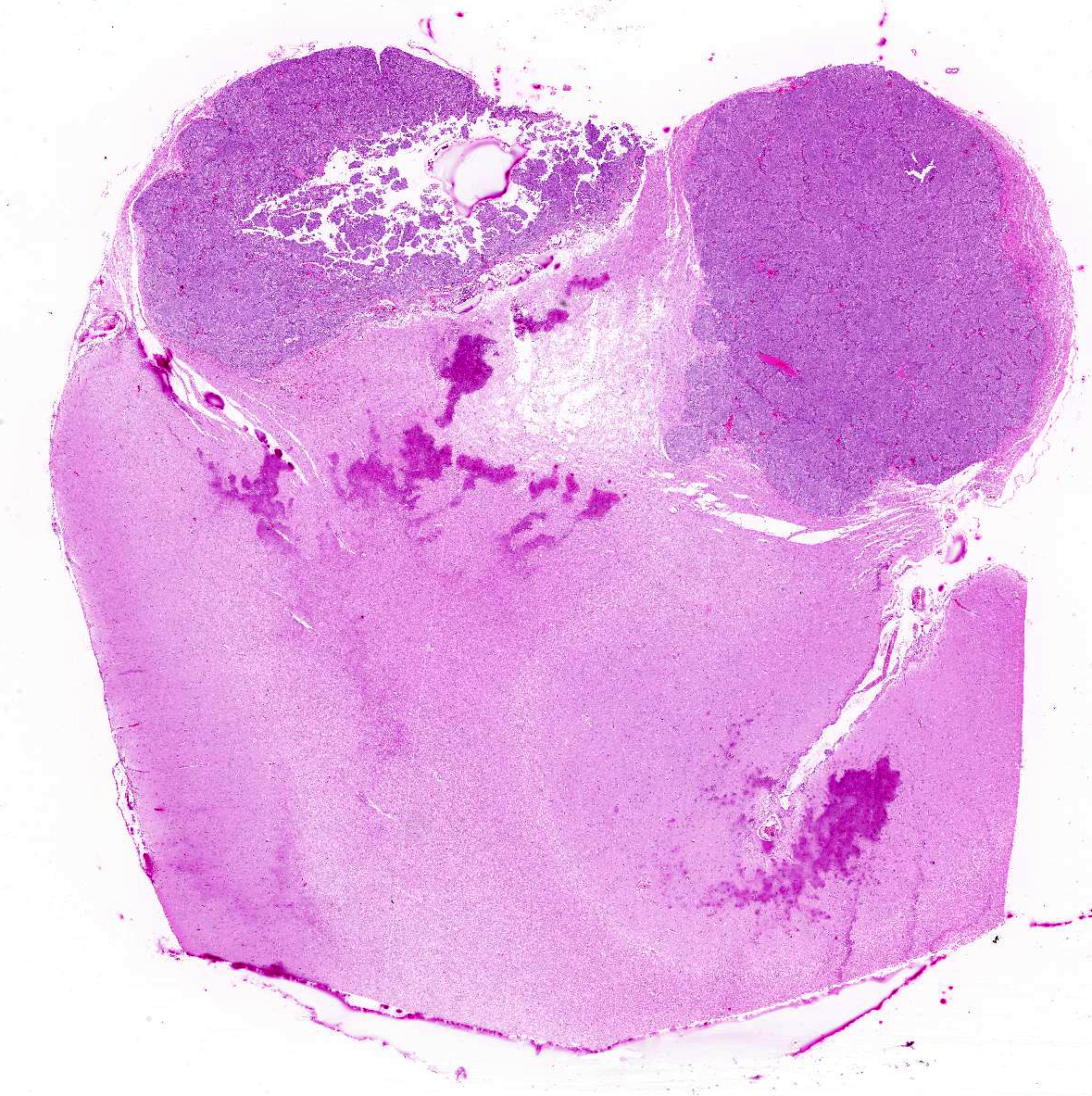
Gross Description:
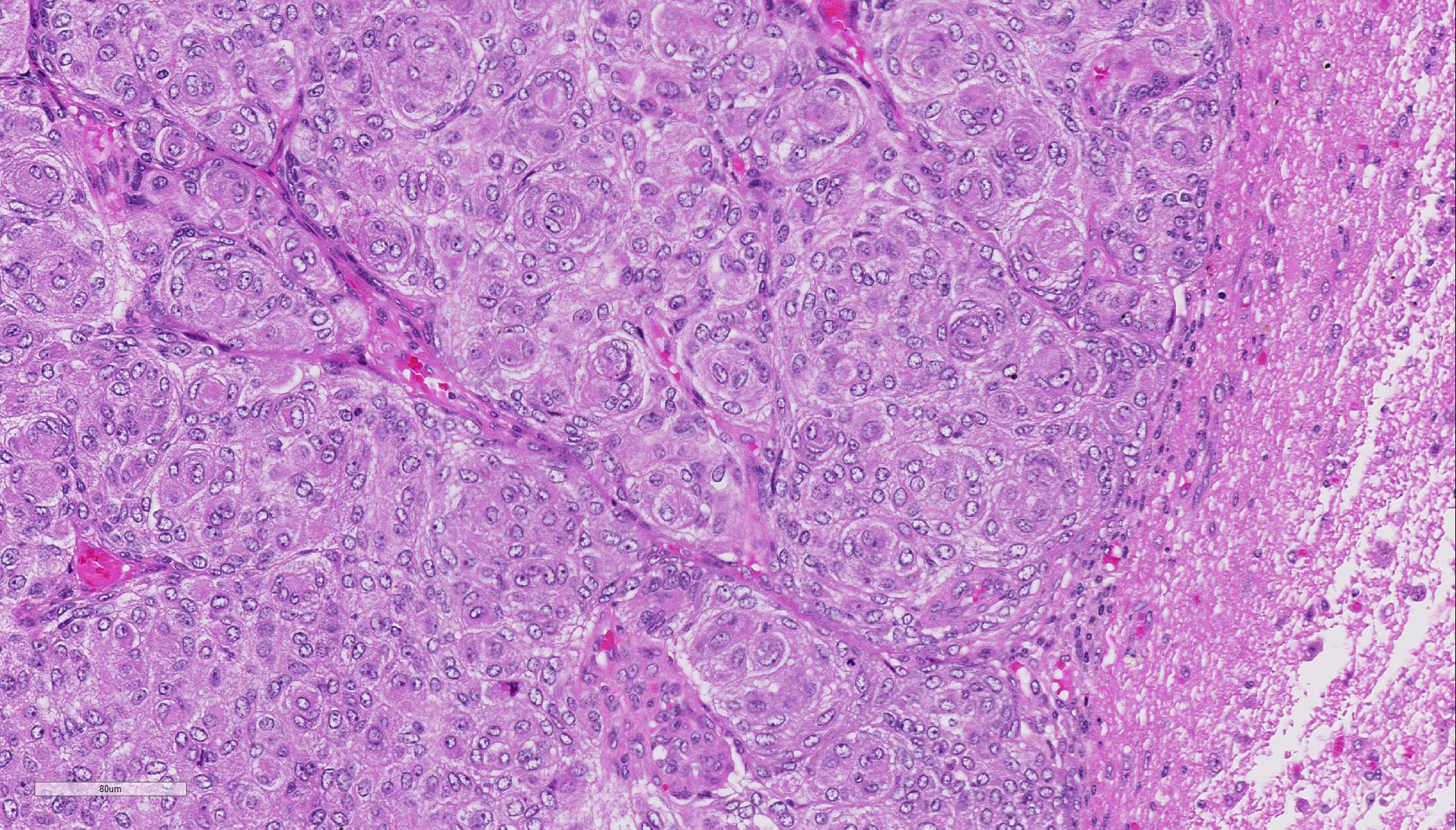
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Older dogs may have a predisposition for primary brain tumors. Meningiomas occur in dolichocephalic breeds, especially German shepherds, golden retrievers and Labrador retrievers, with no consistent sex predisposition.7,9 Meningiomas may be present especially in the rostral cerebrum for a prolonged period before clinical signs of intracranial disease become obvious to the owner. Further, owners may attribute more subtle signs of forebrain disease (such as pacing or mentation change) to the normal aging process in a geriatric pet.5 The majority of animals with brain tumors present with a variety of mild or ill-defined neurological signs.
Canine meningiomas clinically display variable behavior that currently makes outcome difficult to predict. The behavior of canine meningiomas varies widely from case to case. Individual cases receiving basic therapies (corticosteroids and supportive therapies, such as anticonvulsant drugs) can have a much better outcome than other individuals that undergo more aggressive therapeutic regimens, such as surgery and post-operative radiation.1 Meningiomas usually grow as well-demarcated, often lobulated, firm, granular masses that usually have a broad-based or pedunculated attachment to the overlying meninges. Less commonly they can form plaque-like masses over the meninges.3,7 In some cases, canine meningioma shows a large cystic cavity or aggregation of severely vacuolated neoplastic cells as a consequence of ischemic events.7 Meningiomas are gray, sometimes yellow on cut surface, firm, and may be gritty. These tumors grow either by compression or, less commonly, by infiltration of the adjacent brain. In dogs, meningiomas are found commonly in the region of the olfactory bulb and frontal lobes, but can occur anywhere over the surface of the cerebral hemispheres. Supratentorial meningiomas are more common over the meninges of the convexities than in basilar sites.3
Due to the embryonic origin of the meninges, meningiomas exhibit highly variable morphological and immuno-phenotypic patterns.7 A classification based mainly on the WHO classification of human histological subtype describes the following histological patterns: meningothelial, fibroblastic, transitional (mixed), psammo-matous, angiomatous, papillary, granular cell, myxoid, and anaplastic. All of these histological variants except anaplastic (malignant) meningiomas have similar biologic behavior. They are slow growing and cause clinical signs by compression of underlying nervous tissue.5 Areas of chondroid, osseous, myxoid, and xanthomatous-like tissue can be found in the meningothelial and transitional forms.3 Most intracranial meningiomas are benign as indicated by low frequency of metastases and failure to invade the brain. By these criteria, ~ 2.5% are malignant and may metastasize. In contrast, extracranial meningiomas, which occur mainly in the paranasal region and orbit, are anaplastic and locally aggressive.7,9,10
In some cases, it can be difficult to distinguish meningiomas from other neoplasms, such as astrocytomas, oligo-dendrogliomas, metastatic carcinomas, germ cell tumors, and peripheral nerve sheath tumors. In these cases, a basic immuno-histochemical panel consisting of vimentin, CD34, and E-cadherin has been proposed for the characterisation of canine and feline meningiomas. 7 Meningiomas also stain sparse to moderate positively for cyto-keratin, NSE, and S-100 and negative for synaptophysin and GFAP.10
JPC Diagnosis:
Conference Comment:
With the exception of the less common invasive and atypical grade II and malignant and anaplastic grade III types, meningiomas rarely invade into the neuroparenchyma.2,3 Instead, they will grow expansively within the cranium, as in this case, causing compression necrosis of the neuropil.2,3,8 Canine meningiomas bear a striking similarity in both histomorphology and biologic behavior to human meningiomas. As a result, there has been widespread application of the World Health Organization (WHO) classification scheme for humans in scientific reports of canine meningiomas.2-6,8 Conference participants discussed the nine histologic types of meningiomas, including: meningothelial, fibrous (fibroblastic), transitional (as in this case), psammomatous, angiomatous, papillary, granular cell, myxoid, and anaplastic. Other than the anaplastic type, all are slow growing and have a relatively benign biologic behavior.4,5,8 However, regardless of the histomorphology, in meningiomas in domestic animals, malignancy is still based on mitotic rate, cellularity, growth pattern, and tumor invasion into the underlying nervous tissue.2-5,8
Atypical, choroid, and clear cell types are subcategories of grade II meningiomas. Grade II meningiomas require 4 mitoses/ 10 high powered fields and at least three of the following features: of architectural pattern replaced by sheets of cells, small cell formation with a high nuclear: cytoplasmic ratio, nuclear atypia or macronuclei, hypercellularity, and spontaneous necrosis.2,3 The clear cell category is characterized by sheets of epithelial cells with abundant PAS positive cytoplasm, indicating glycogen accumulation. Grade III malignant meningiomas are comprised of papillary, anaplastic, and and rhomboid types and are characterized by extreme cellular anaplasia, >20 mitoses/10 high powered fields, necrosis, and neuroinvasion.3
Conference participants discussed doublecortin (DCX), Ki-67, E-cadherin, N-cadherin, and beta-catenin as potential immunohistochemical (IHC) stains that would help differentiate malignant from benign meningiomas. A relatively recent study in Veterinary Pathology used the previously mentioned IHC stains to predict the biologic behavior of meningiomas.4 The authors found that doublecortin (DCX), which is important in neuroblast migration during development, is highly expressed in invasive brain tumors, including malignant meningiomas. Additionally, Ki-67 staining, a standard marker of cell proliferation, is significantly higher in anaplastic meningiomas compared to benign varieties and is widely used to evaluate tumor grade and malignancy in humans.4 The authors also found that there is positive correlation between DCX and N-cadherin expression and conversely a negative correlation between E-cadherin and N-cadherin expression. Additionally decreased E-cadherin expression is associated with increased nuclear beta-catenin expression suggesting that decreased E-cadherin, increased N-cadherin, DCX expression, and nuclear beta-catenin staining are all associated with malignant meningiomas.4,8
References: