Signalment:
10 to11-month-old, male and female, Beagle dogs (
Canis familiaris).These dogs were part of the high dose group involved in a 7-day study with an orally administered experimental drug. Dogs were dosed once daily and euthanized on day 8. The animal care and experimental procedures of this study were conducted in compliance with the U.S. Animal Welfare Act and were performed in accordance with the standards of the Institute of Laboratory Animal Resources (ILAR) Guide (1996). The Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International accredited the facility in which this study was conducted.
Gross Description:
In the heart of a few animals in the high dose group, there were multifocal areas of red or pale discoloration.
Histopathologic Description:
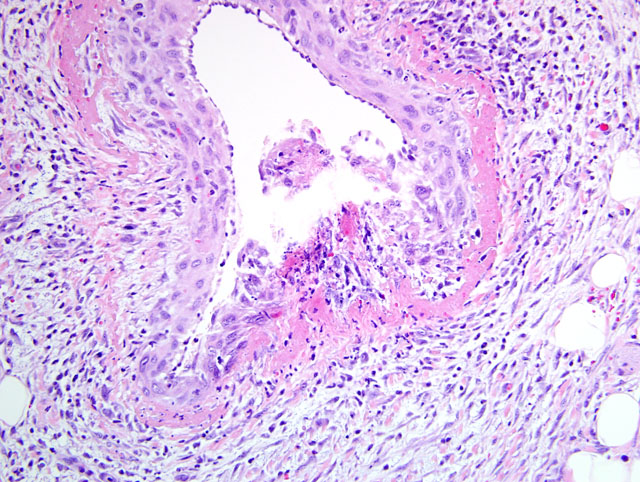
The tunica media of multiple small- to medium-sized arteries in the epicardium and myocardium is segmentally to circumferentially replaced by brightly eosinophilic, homogenous material (fibrinoid necrosis), with small to moderate numbers of degenerate neutrophils and histiocytes, scattered karyorrhectic debris, and occasionally hemorrhage (
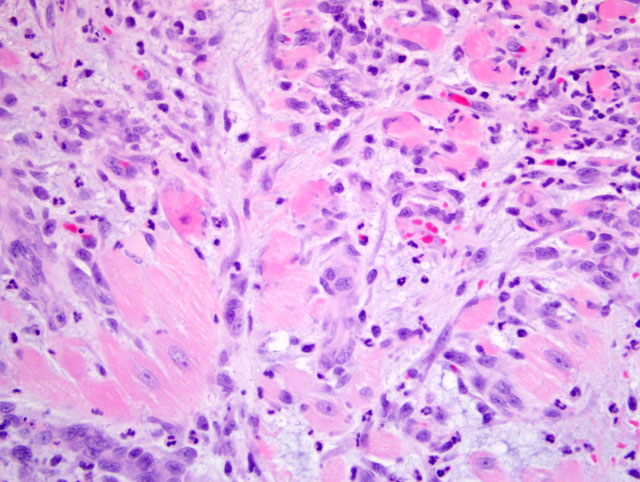
figs 1 and 2). Smooth muscle fibers of the tunica media in some arteries contain plump, vesicular nuclei. There is moderate expansion of the tunica adventitia by edema and inflammatory cells. Multifocally in the myocardium, particularly the atria, there are foci of interstitial expansion by edema with loose infiltration by small numbers of neutrophils, histiocytes and lymphocytes. Scattered cardiomyofibers in affected areas are shrunken and hypereosinophilic, and often surrounded by cells with large, vesicular nuclei (
fig 3). Capillaries in affected areas are lined by plump endothelial cells. In some sections, there is multifocal subendocardial and/or subepicardial hemorrhage.Â
Morphologic Diagnosis:
Heart:
1) Severe, multifocal, subacute, necrotizing arteritis with myocardial degeneration and necrosis.Â
2) Minimal, multifocal, subendocardial and subepicardial hemorrhage (some sections).
Lab Results:
Hematologic alterations in the high dose group included decreased red blood cell count, hemoglobin, and hematocrit with an increased white blood cell count due to elevated neutrophils and monocytes. Clinical chemistry perturbations in the high dose group included increases in troponin and total bilirubin.
Condition:
Phosphoesterase inhibitor toxicosis
Contributor Comment:
This case represents an example of drug-induced vascular injury (DIVI) due to administration of a phosphodiesterase inhibitor in the dog. DIVI is currently a topic of debate in regulatory circles, as several drugs are associated with vascular injury in laboratory animals without concordant vascular injury in humans. In laboratory beagles, compounds that cause DIVI often have a predilection for the coronary vascular bed. This vascular bed is also a common area for spontaneous arterial lesions; therefore, care must be taken when interpreting vascular changes.(1,5) Manifestations of DIVI in laboratory beagles include vasoactive arteriopathy (produced by some vasodilator/positive inotropic and vasoconstrictor compounds), toxic vasculitis, and hypersensitivity vasculitis.(1)
Vasodilator and/or positive inotropic drugs (e.g. minoxidil, phosphodiesterase inhibitors, theobromine, others) produce distinctive, time-dependent, arterial changes affecting primarily extramural and intramural, small to medium-sized arteries. Gross changes include epicardial and possibly myocardial petechial and ecchymotic hemorrhages. At 7 days of treatment or less, microscopic changes include necrosis of the tunica media and hemorrhage, with inflammation and perivascular edema. Intimal and adventitial proliferative changes are observed with more chronic treatment.(1,4) Distinct changes associated with administration of phosphodiesterase III inhibitors to dogs include coronary arteriopathy generally restricted to extramural coronary arteries, myocardial necrosis with endocardial hemorrhage, and atrial hemorrhage with formation of granulation tissue.(1,3)
Vasoconstrictive agents (e.g. endothelin-1, digoxin, others) induce arterial changes consisting of medial thickening and necrosis, with hyalinization after longer-term treatment, and tend to affect small caliber arteries in a variety of organs. Some compounds (e.g. allylamine, systemic administration of monocrotaline and mitomycin C) may act directly on the arterial wall (endothelium and/or smooth muscle) causing toxic necrotizing vasculitis characterized by changes varying from acute necrosis of the tunica media with infiltration by neutrophils to medial scarring and intimal/adventitial fibrosis. Some drugs have been reported to cause immune-mediated disease due to Type III and, more commonly, Type IV hypersensitivities. Arterial changes associated with the Type IV hypersensitivity are not confined to coronary vascular beds, and are characterized by infiltration with mononuclear cells, possibly mixed with eosinophils, without medial necrosis.(1,4,5)
The pathogenesis of DIVI due to vasoactive compounds is still not clear. Vasodilator/positive inotropic agents produce their pharmacologic effects via a variety of mechanisms, including: direct relaxation of arterial smooth muscle, opening of potassium channels, inhibition of vascular smooth muscle phosphodiesterase, or blockage of endothelin receptors. The most frequently cited mechanism for the pathologic changes incurred by vasodilator/positive inotropic agents is vasodilation -> increased blood flow -> turbulence, altered shear stress -> homeostatic imbalance of endothelial cells -> injury.(2) Arterial changes due to vasoconstrictor agents are attributable to local and/or systemic hypertension produced by these compounds.(5)
JPC Diagnosis:
Heart, epicardial and myocardial arteries: Arteritis, proliferative and necrotizing, multifocal, marked, with multifocal myocardial degeneration and necrosis.Â
Conference Comment:
Because the submitted slides are from multiple dogs, there is some section variation. Some slides feature reactive mesothelium overlying the epicardial surface and/or serous atrophy of pericardial fat.Â
The contributor provides an excellent synopsis of this entity. Of immense practical concern is the differentiation of drug-induced from spontaneous vascular injury in general, and idiopathic polyarteritis of beagle dogs in particular. While many features overlap, there are some diagnostically useful differences between DIVI and idiopathic polyarteritis. The clinical signs in DIVI are variable and likely unrelated to vascular effects, while in idiopathic canine polyarteritis there is generally fever, weight loss, cervical neck pain (thus the clinical term, beagle pain syndrome), and neutrophilic leukocytosis. In DIVI, vascular lesions are generally restricted to coronary arteries, while in idiopathic canine polyarteritis multiple organs are affected. While the histologic lesions of the vasculature may be similar in these entities, idiopathic canine polyarteritis generally exhibits mononuclear periarterial to transmural inflammation, a feature which is absent or only mild in DIVI. Finally, atrial hemorrhage is characteristic of DIVI, but not idiopathic canine polyarteritis.(1)
References:
1. Clemo FAS, Evering W, Snyder PW, Albassam MA: Differentiating spontaneous from drug-induced vascular injury in the dog. Toxicol Pathol
31(Suppl.):25-31, 2003
2. Enerson BE, Lin A, Lu B, Zhao H, Lawton M, Floyd E: Acute drug-induced vascular injury in beagle dogs: pathology and correlating genomic expression. Toxicol Pathol
34:27-32, 2006
3. Joseph EC: Arterial lesions induced by phosphodiesterase III (PDE III) inhibitors and DA1 agonists. Toxicol Lett
112-113:537-546, 2000
4. Louden C, Brott D, Katein A, Kelly T, Gould S, Jones H, Betton G, Valetin J, Richardson R: Biomarkers and mechanisms of drug-induced vascular injury in non-rodents. Toxicol Pathol
34:19-26, 2006
5. Louden C, Morgan DG. Pathology and pathophysiology of drug-induced arterial injury in laboratory animals and its implications on the evaluation of novel chemical entities for human clinical trials. Pharmacol Toxicol
89:158-170, 2001