Signalment:
Gross Description:
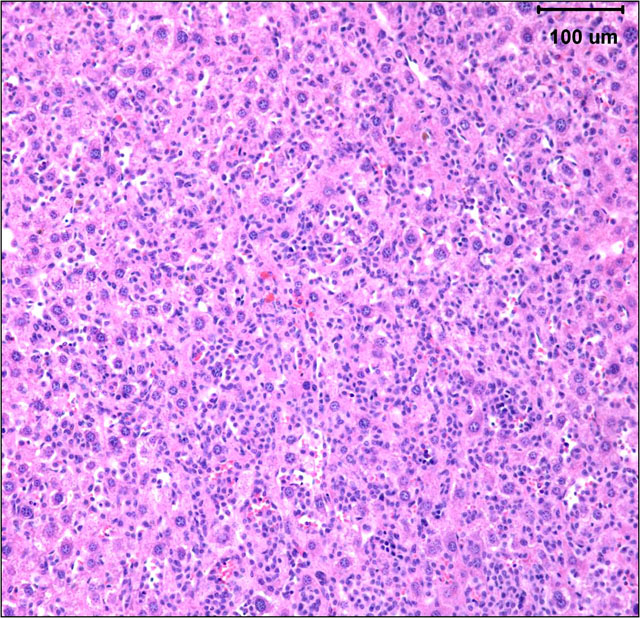
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
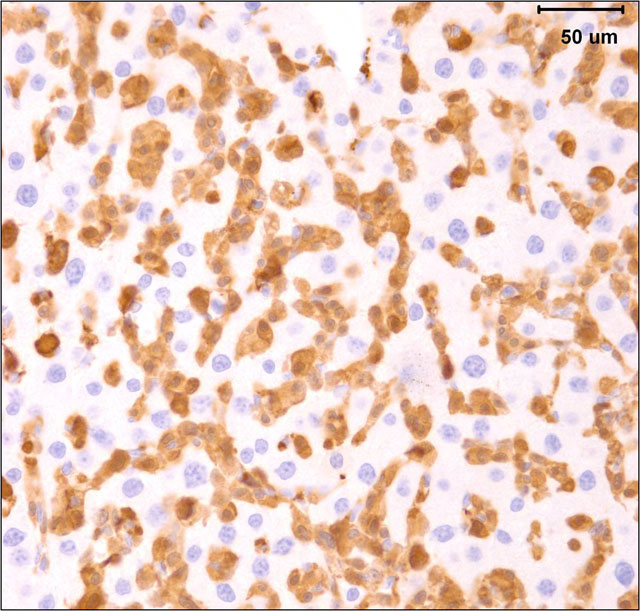
The gross and microscopic appearance of HS depends on organs involved. The liver involvement is the most common manifestation of HS regardless of the mouse strain.7 The liver with HS is severely, diffusely enlarged and without focal lesions. Histologically, tumor cells are present diffusely or multifocally within sinusoids and vascular spaces. Progressive growth of neoplastic cells leads to compression of hepatic cords and hepatocyte atrophy.(3, 7) Uterine involvement is strongly strain-dependent in mice: uterine HS is rare in C57BL/6 mice but common in CBA mice.(7) HS in uterus may present as diffuse thickening of both horns or as 1 to several variably sized nodules. Histologically, neoplastic histiocytic cells that infiltrate uterine wall tend to be elongated to fusiform.(3, 7) Erythrophagocytosis may be associated with the neoplastic infiltrates, particularly in the liver and multinucleated giant cells may be present in some tumors.(3, 7) Immunohistochemical stains that aid identification of neoplastic cells as histiocytes include Mac-2, F4/80, and lysozyme.(9)
Recent publications have linked development of HS in the liver in C57BL6/J mice with concurrent hepatic (but not splenic) extramedullary hematopoiesis (EMH) and hematologic abnormalities such as anemia.(1, 5) At this point it is not clear whether HS and hepatic EMH are co-incidental lesions or if one of them leads to the other. Concurrent hematologic abnormalities may suggest genetic abnormality in myeloid stem cells.
The submitted case is from a mouse with homozygous, single base mutation in Mcm4 gene known as Chaos3. This mutation induced high incidence of mammary adenocarcinomas in C3H mice.(7) In contrast, C57BL/6 mice with Chaos3 mutation have high prevalence of histiocytic sarcoma with shortened tumor latency of less than 12 months. Diffuse liver involvement is noted most commonly in these mice but nodular tumor infiltrates and marked destruction of hepatic parenchyma by HS is seen occasionally. All mice with hepatic HS have extramedullary hematopoiesis in the liver and marked erythroid hyperplasia in the spleen. Some mice have intraabdominal solid masses in peripancreatic omentum and elsewhere diagnosed as HS based on presence of Mac-2 positive cells with histiocytic appearance (spindle to polygonal cells, abundant cytoplasm, and oval to indented nucleus) and neoplastic features (moderate mitotic figure rate and bizarre mitoses).
Gene complex MCM2-7 which includes MCM4 encodes protein complex that is recruited to DNA replication origins and ensures a singe initiation of DNA synthesis during S phase restricting genome replication to once per cell cycle.(7) Chaos3 mutation was first identified in the screen for chromosome instability. High incidence of tumors and short tumor latency periods in mice with Mcm4Chaos3/Chaos3 suggests that genomic instability may have a causative role in cancer. It is not clear at this point yet whether C57BL/6 mice with Mcm4Chaos3/Chaos3 genotype have hematologic abnormalities such as anemia concurrently with HS.
JPC Diagnosis:
2. Liver, hepatocytes: Microvesicular lipidosis, multifocal, moderate.
Conference Comment:
In rats, the most commonly affected strain is Sprague Dawley, and tumors are generally seen in animals over 12 months of age with no gender preference. The organs affected in rats are similar to those in mice; the most common sites are liver, lymph nodes, spleen, mediastinum, retroperitoneum, and subcutaneous tissue. Affected rats most commonly have nodules of tumor cells that displace normal organ parenchyma, whereas in the mouse liver, neoplastic cells typically infiltrate sinusoids with no distinct mass formation.(6)
Histologically, tumor cells are pleomorphic with vesicular nuclei, prominent nucleoli, and abundant cytoplasm. Multinucleate giant cells are a common component of tumors with a predominantly histiocytic makeup. Because of the variable morphology of neoplastic histiocytes, the differential diagnostic list includes various sarcomas, lymphoma and granulomatous inflammation.(6)
References:
2. Blackwell BN, Bucci TJ, Hart RW, Turturro A: Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol. Pathol. 23: 570-582, 1995
3. Frith CH: Histiocytic sarcoma, mouse. In: Monographs on Pathology of Laboratory Animals. Hematopoietic system, Jones TC, Ward JM, Mohr U, Hunt RD (eds). Springer-Verlag, Berlin, Heidelberg, pp 58-65, 1990
4. Frith CH, Ward JM, Chandra M: The morphology, immunohistochemistry and incidence of hematopoietic neoplasms in mice and rats. Toxicol. Pathol. 21: 206-218, 1993
5. Lacroix-Triki M, Lacoste-Collin L, Jozan S, Charlet JP, Caratero C, Courtade M: Histiocytic sarcoma in C57BL/6J mice is associated with liver hematopoiesis: Review of 41 cases. Toxicol. Pathol. 31: 304-309, 2003
6. Percy DH, Barthold SW: Pathology of Laboratory Rodents and Rabbits, 3rd ed., pg. 116, 170-171, 172. Blackwell Publishing, Ames, Iowa, 2007
7. Shima N, Alcaraz A, Liachko I, Buske TR, Andrews CA, Munroe RJ, Hartford SA, Tye BK, Schimenti JC: A viable allele of Mcm4 causes chromosome instability and mammary adenocarcinomas in mice. Nature Genetics 39: 93-98, 2007
8. Turusov VS: Histiocytic sarcoma. IARC Sci. Publ. 111: 671-680, 1994
9. Ward JM, Sheldon W: Expression of mononuclear phagocyte antigens in histiocytic sarcoma of mice. Vet. Path. 30: 560-565, 1993