Signalment:
20-year-old
female Indian rhesus macaque (
Macaca mulatta).This
animal had 2 months of lethargy, large clitoris, pain/discomfort vaginal
bleeding. The body weight lost 3 kg in 2 months. A large firm mass was palpable
in the lower abdomen, mainly on left side. The mass was round, measuring 7 x7
cm. Ultrasound revealed large mass with multiple round cavities inside. The
animal developed a moderate regenerative anemia. Although extensive care and
treatment were given, euthanasia was elected due to the grave prognosis.
Gross Description:
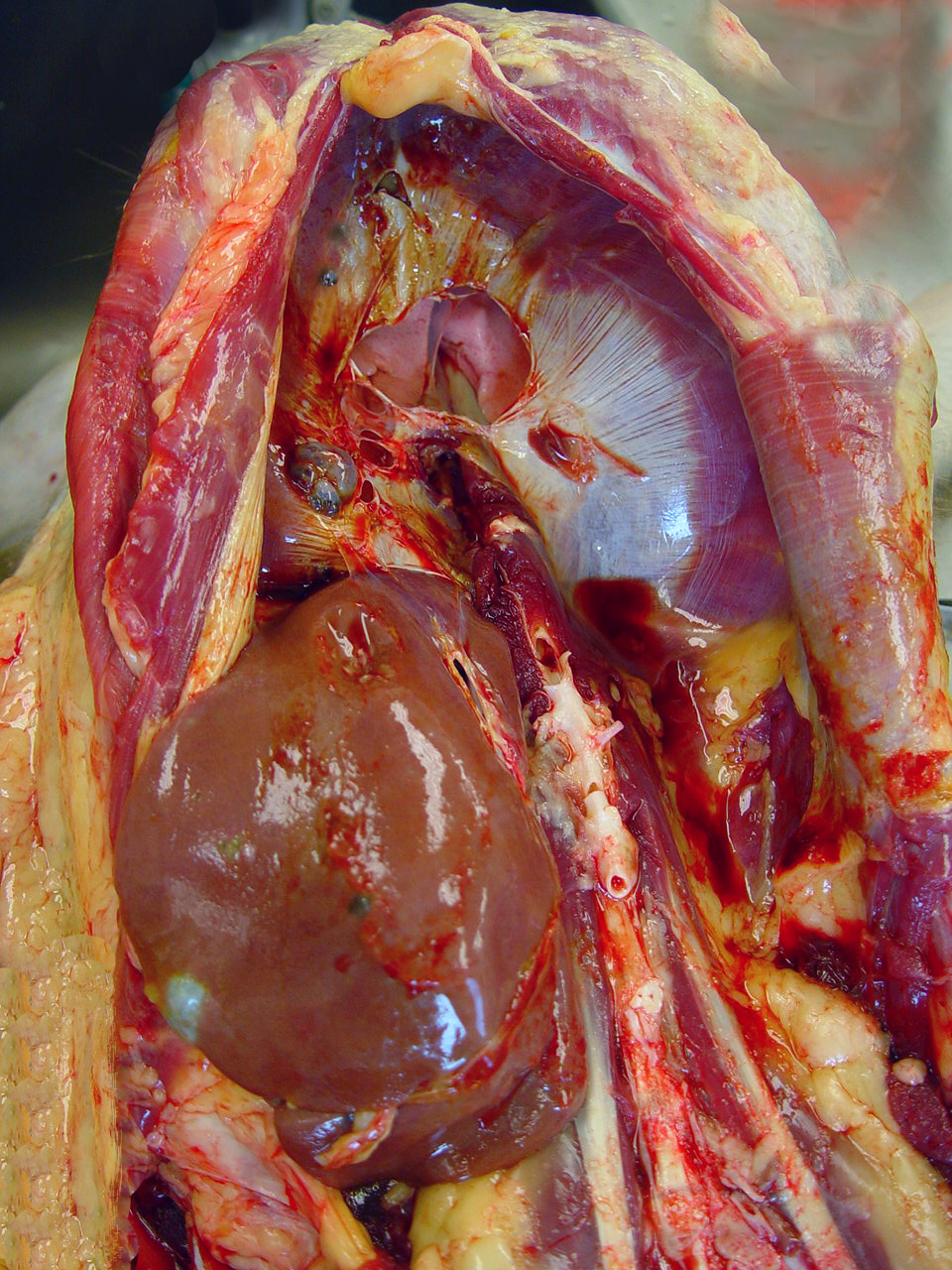
Presented was a
thin, dehydrated aging animal. A large, firm mass was palpated at the lower
abdomen. The subcutaneous tissue over the abdominal mass was dark-red and
edematous. Extending from the uterus to the abdominal wall and incorporating
with ovaries, ovary ducts, and serosa of the colon was a 15 -20 cm in diameter,
dark-red cystic masses, which contained a large amount of dark-red fluid on the
cut section. Multifocal variable-sized, dark-red cystic masses are also noted
on the diaphragm, liver, and mesentery. The central tendon of the diaphragm was
fragile and easily pierced by force. Other findings included severe thymic
atrophy and mild, bilateral hydronephrosis.
Histopathologic Description:
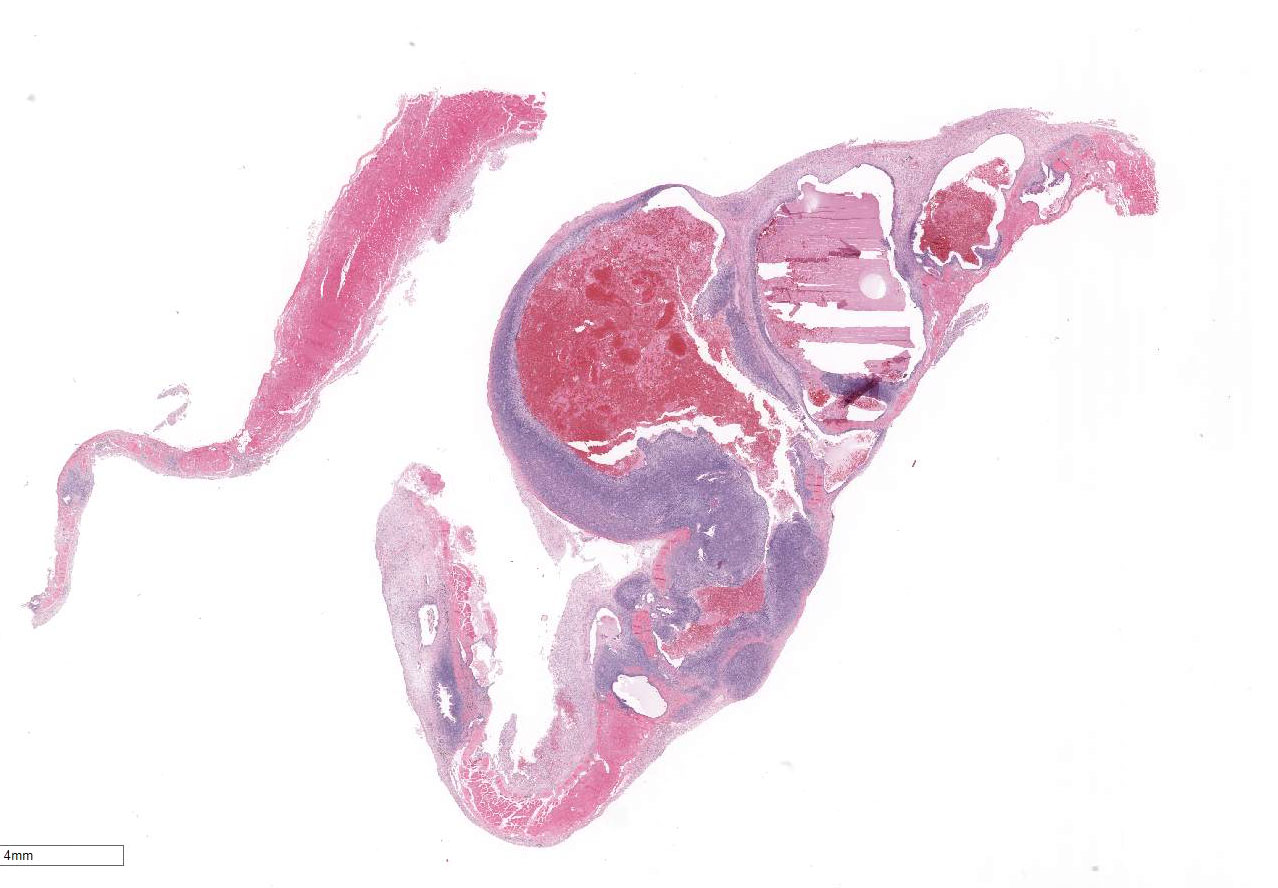
Expanding
and disrupting the diaphragm are multiple, unencapsulated masses composed of
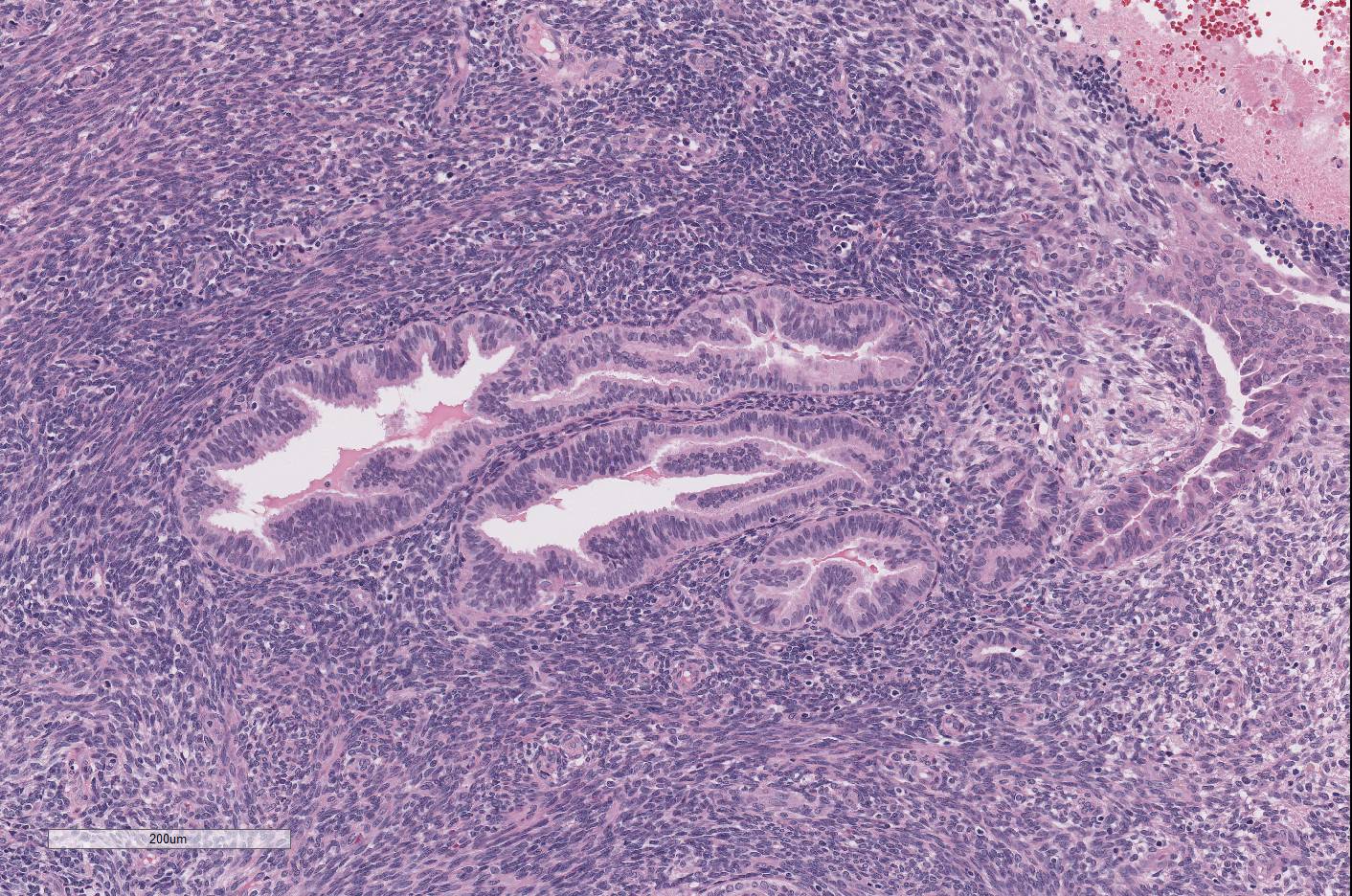
variable tortuous endometrial glands surrounded by abundant, densely cellular
endometrial stroma. The endometrial glands are generally lined by simple
columnar, ciliated epithelial cells. Some parts of glands are lined by
flattened to pseudostratified cells. Occasionally the epithelial cells form
islands or papillary projections in the lumen. The epithelial cells have
indistinct cell borders, a moderate amount of eosinophilic cytoplasm, and
prominent basilar nuclei. Nuclei are round to oval with finely stippled
chromatin and 1-2 prominent nucleoli. There are large amounts of amorphous,
eosinophilic material and admixed with numerous erythrocytes. The endometrial
stroma is composed of spindle cells with indistinct cell borders, scant
eosinophilic, fibrillar cytoplasm and an oval to elongate nucleus with finely
stippled chromatin. The mitotic figures
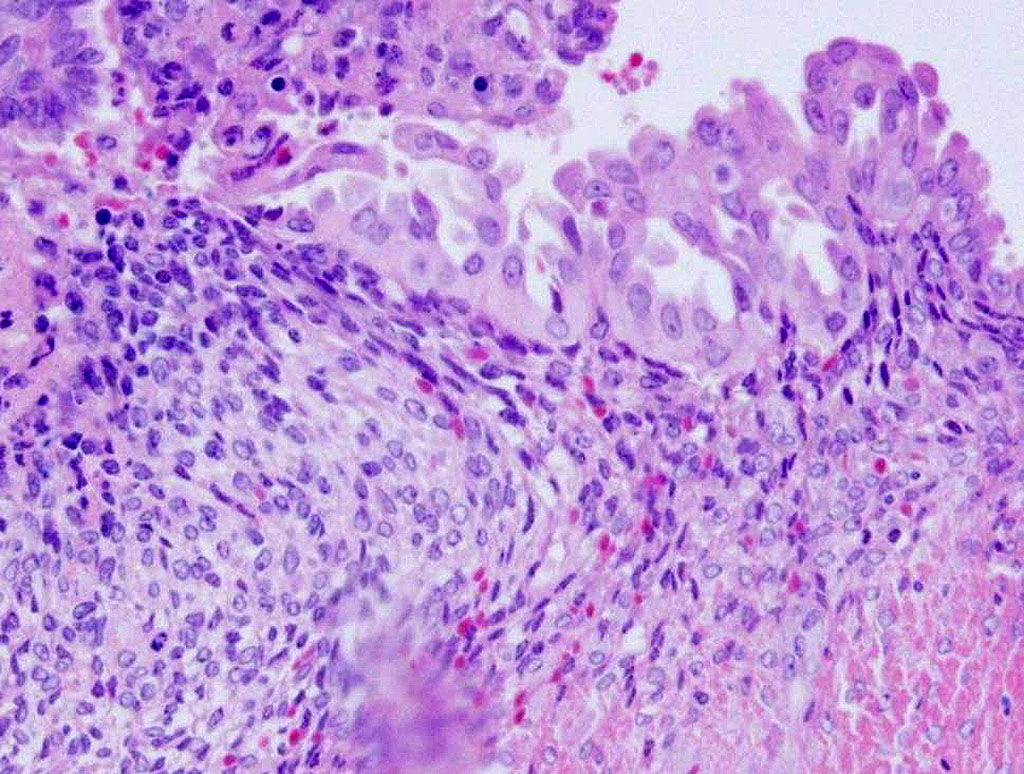
are 1-2/HPF in both glandular epithelium and stroma. Diffusely the
serosa is markedly expanded by reactive fibroblasts, edema, hemorrhage, and
many lymphocytes, plasma cells, and macrophages. Some macrophages contain
abundant cytoplasmic brown pigment (hemosiderosis). Multifocally the muscle
adjacent to the masses showed variable degeneration and necrosis.
Morphologic Diagnosis:
Diaphragm, Endometriosis.
Lab Results:
N/A
Condition:
Diaphragmatic endometriosis
Contributor Comment:
Endometriosis
usually occurs in the pelvis and the most commonly involved organs are ovaries,
uterosacral and broad ligaments, and parietal pelvic peritoneum. Diaphragmatic
endo-metriosis is rare, often asymptomatic, and always associated with severe
pelvic involvement.
4,7 Ectopic endometriosis has also been reported
in umbilicus, skin, vagina, vulva, cervix, inguinal canal, upper abdominal
peritoneum, liver, spleen, gastrointestinal tract, urinary system, breasts,
pleural cavity, brain, eye, lymph nodes, lung and pericardium in human.
4
Pleural and lung endometriosis have been reported in an aged rhesus macaque1
and sooty mangabey 2009 conference
19, case 01 respectively. Diaphragmatic
endometriosis, like in this case, can involve entire thickness of the muscle.
It is common to extend into pleural space in human, but not presented in this
case.
4 The pathogenesis of endometriosis has
been well discussed in the previous conferences 2011 conference
11, case 01 and 2009 conference
19, case 01. The primary theory of Sampsons three-fold transplantation likely applies to
this case. The retrograde regurgitation of endo-metrial cells passes through
the oviducts into the peritoneal cavity and proliferates in ectopic sites.
8
JPC Diagnosis:
Diaphragm:
Endometriosis, rhesus macaque,
Macaca mulatta.
Conference Comment:
Endometriosis is characterized by the presence of well-differentiated, viable,
and hormone responsive endometrial glands and stroma outside the uterus.
1,4,5,7
This is one of the most common gynecologic diseases encountered in female
humans as well as Old World primates.
1 There have been rare reports
of endometriosis in the elephant shrew, long-tongued bat, and one case report
of an endometrioma in a dog.
3,7 The
most widely studied animal model for endometriosis in humans is the baboon. The
prevalence of endometriosis in captive female baboons is as high as 20%. It is
thought that baboons in captivity develop endometriosis at a higher rate than
wild baboons because of regular menstrual cycles without intervening pregnancy
in this population of animals.
7 The vast majority of endometriosis
cases in Old World primates occurs within the abdominal cavity and is grossly
identifiable as blood-filled chocolate cysts which can progress to fibrotic
scars in chronic cases. Typically, endometriosis demonstrates positive
immunoreactivity for pancytokeratin, vimentin, estrogen receptor, and pro-gesterone
receptor.
1
In this case, several conference
participants noted that the endometrial glands are filled with abundant
hemorrhage, consistent with active menses in this animal. The endometrial
tissue in endometriosis is responsive to cycling estrogen and pro-gesterone,
and therefore will undergo cycles of proliferation and degradation in response
to the normal menstrual cycle.
1,7,8 Hemorrhage associated with
endometriosis can be severe enough to cause anemia and can rarely rupture
causing hemorrhagic ascites.
1,2,7,8 Conference participants
discussed various risk factors for the development of endometriosis in
non-human primates. These include chronic un-interrupted estrus cycles
throughout life, resulting in increased endometrial turnover compared to
multiparous primates. Females older than ten years with an affected
first-degree relative are also predisposed. Non-laparoscopic abdominal surgical
procedures, including hysterectomy and cesarean section, are also implicated,
in addition to estradiol implants.
1,5 The moderator also discussed
that aged non-human primates are much more likely to develop endometriosis
compared to older women due to lack of menopause in these species.
5
Unfortunately, the reproductive history of this animal was not provided.
The conference
moderator also discussed the importance of distinguishing well-differentiated
endometrial tissue in endometriosis from endometrioid carcinoma. In
endometrioid carcinoma, glands will be irregular with little to no intervening
stroma and demonstrate significant nuclear atypia. Endometrioid carcinoma has
been rarely reported to develop from endometriosis.
1,4 In addition,
multifocally
within this section there are large areas where the endometrial epithelial cell
nuclei are rounded and vesicular with abundant pale vacuolated cytoplasm,
consistent with stromal decidualization and epithelial plaque reaction.
Decidualization of the endometrial glands occurs under the influence of
progesterone and is a response to blastocyst implantation or other trauma to
the endometrial stroma. This is required for maintenance of normal pregnancy in
humans and non-human primates.
2 In endometriosis, it is thought that
this reaction develops secondary to elevated pro-gesterone. Endometrial
decidualization is non-neoplastic, but grossly and histo-logically mimics
malignant neoplastic lesions such as mesothelioma and carcino-matosis. In a
recent article in
Veterinary Pathology, Atkins et al. demonstrates that
the decidualized stroma stained positive for vimentin, CD10, progesterone, and
estrogen consistent with reported deciduosis in humans.
2
References:
1. Assaf BT, Miller
AD. Pleural endometriosis in an aged rhesus macaque (
Macaca mulatta): A
histopathologic and immunohistochemical study.
Vet Pathol. 2012;
49(4):636-641.
2. Atkins HM,
Lombardini ED, Caudell DL, et al. Decidualization of endometriosis in
macaques.
Vet Pathol. 2016; 53:1252-1258.
3. Paiva BH, Silva
JF, Ocarino NM, et al. A rare case of endometrioma in a bitch.
Acta Vet
Scand. 2015; 57:31.
4. Ceccaroni M,
Roviglione G, Rosenberg P, Pesci A, Clarizia R, Bruni F, et al. Pericardial,
pleural and diaphragmatic endometriosis in association with pelvic peritoneal
and bowel endometriosis: A case report and review of the literature.
Wideochir
Inne Tech Maloinwazyjne. 2012; 7(2):122-131.
5. Fazleabas
AT, Brudney A, Gurates B, Chai D, Bulun S. A modified baboon model for
endo-metriosis.
Ann N Y Acad Sci. 2002; 955:308-17.
6. Hastings
JM, Fazleabas AT. A baboon model for endometriosis: Implications for fertility.
Reprod Biol Endocrinol. 2006; 4:1-7.
7. Nezhat
C, King LP, Paka C, Odegaard J, Beygui R. Bilateral thoracic endometriosis
affecting the lung and diaphragm.
JSLS. 2012; 16(1):140-142.
8. Van der Linden
PJ. Theories on the pathogenesis of endometriosis.
Hum Reprod. 1996;
11(3):53-65.