Signalment:
Gross Description:
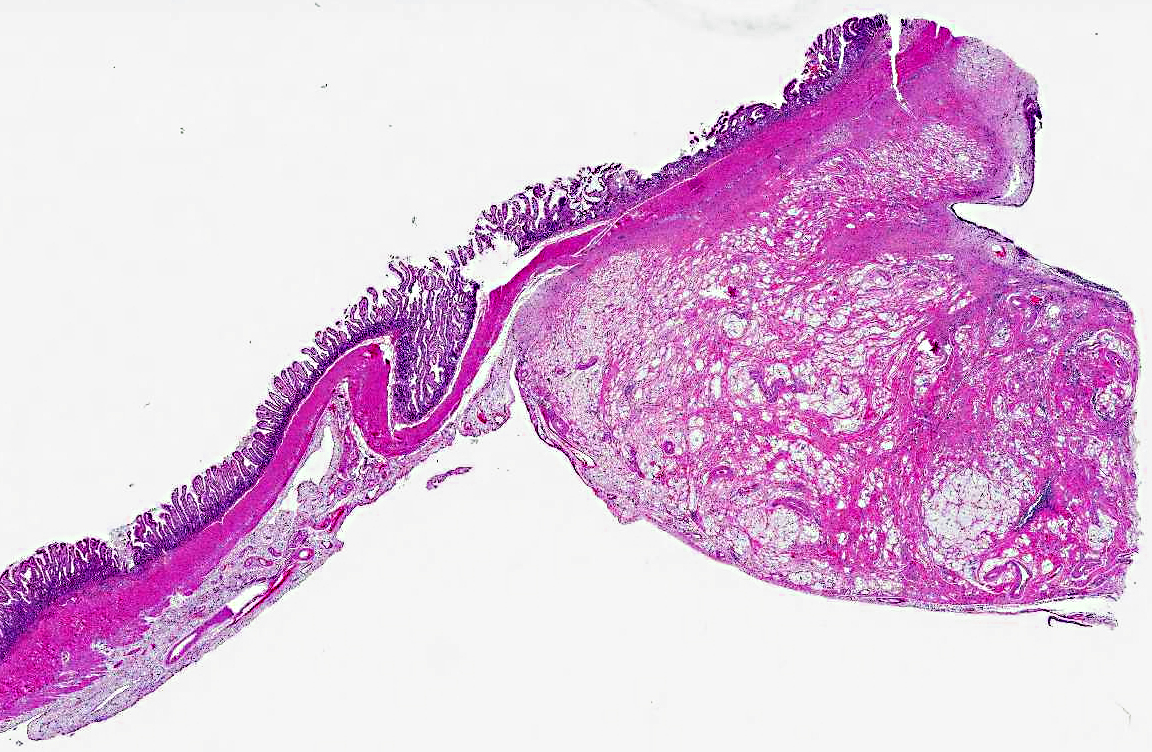
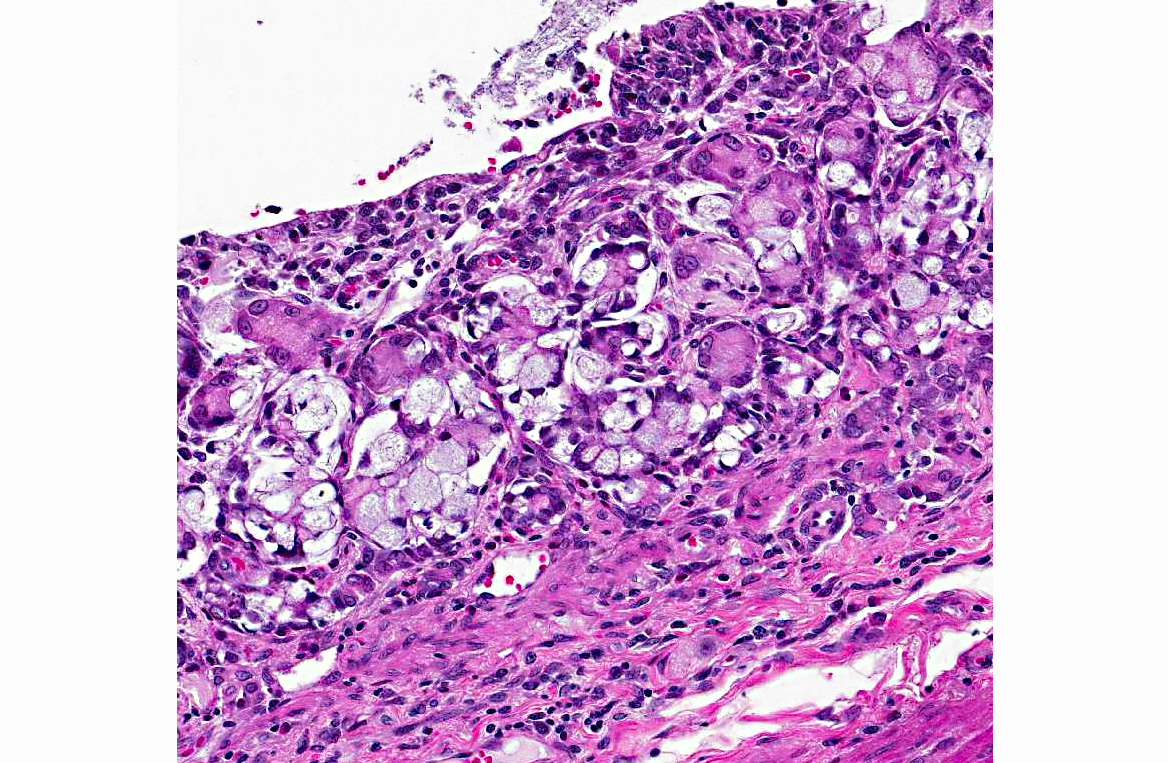
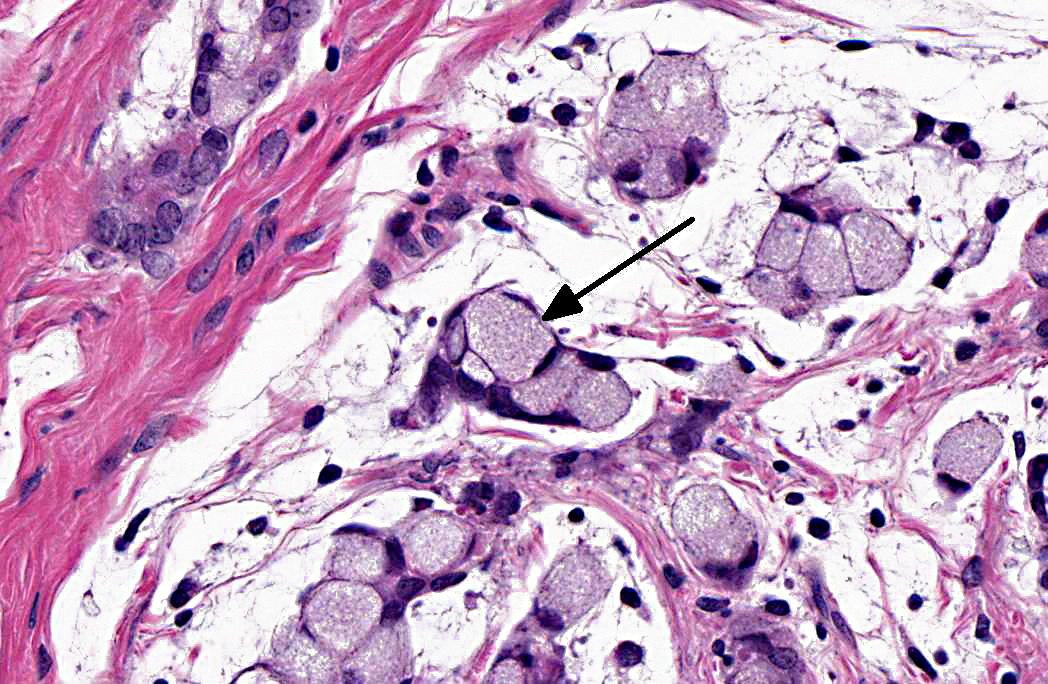
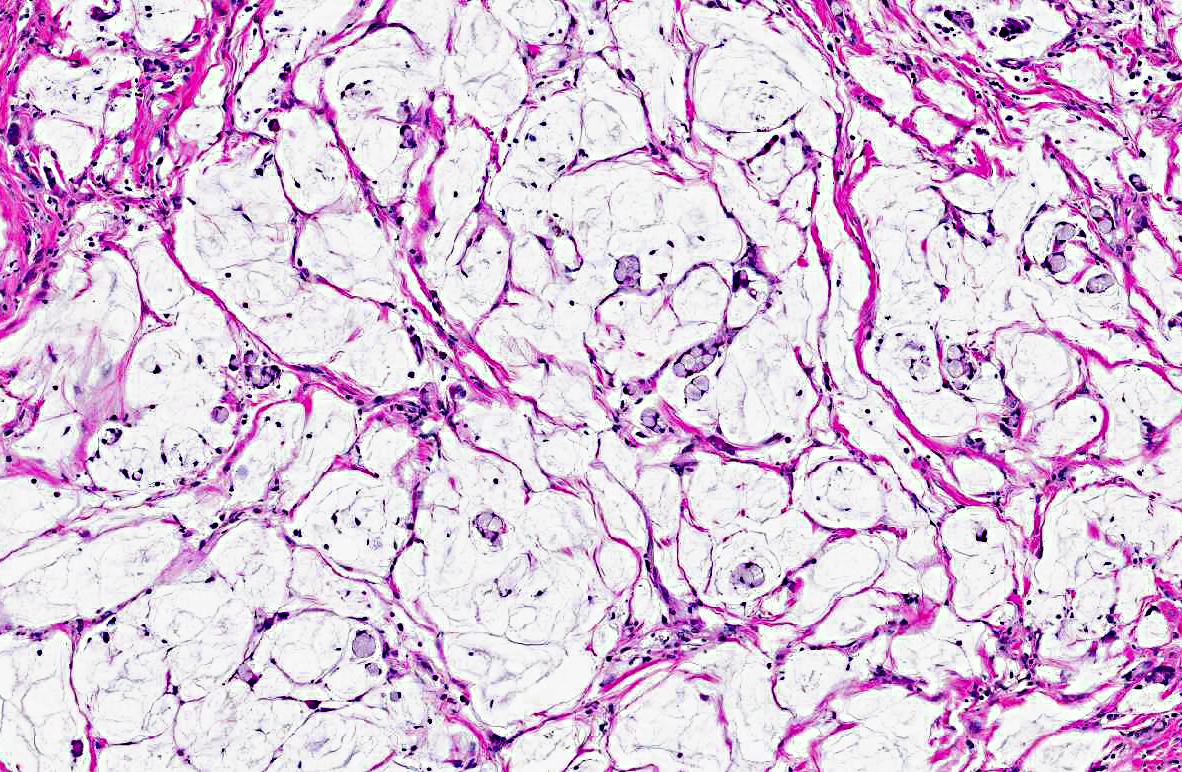
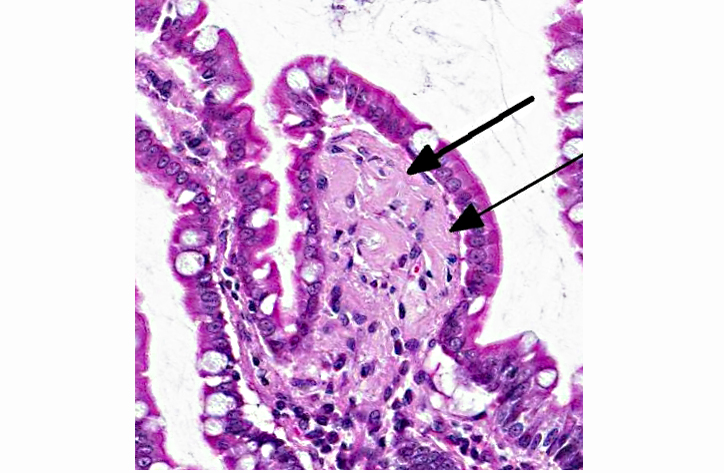
Histopathologic Description:
Morphologic Diagnosis:
1. Jejunum: Adenocarcinoma, mucinous.Â
2. Jejunum, lamina propria: Amyloidosis, multifocal, mild.
Condition:
Contributor Comment:
A recent report suggests that small intestinal adenocarcinoma may be a relatively common neoplasm in aged common marmosets (Callithrix jacchus).(5) In this report, tumors were usually located within the proximal small intestine, immediately distal to the duodenum, with grossly observed thickening and stricture at the tumor site often present. Signet-ring cell differentiation, lymphatic infiltration, and metastatic spread to the regional lymph nodes were other common findings. Carcinomatosis was not observed. An association between presence of callitrichine herpesvirus 3 (marmoset lymphocryptovirus) or Helicobacter sp. and tumor development was not found.(5) This case shares similar features to those reported; however, evidence of metastasis was not observed in histologically examined tissues.Â
The reason for the predisposition of development of small intestinal adenocarcinoma at the duodenal-jejunal interface is unknown; however, there is a belief among some pathologists that components of biliary or pancreatic secretions that enter the intestine at this location may result in cell damage and subsequent tumorigenesis.(1) Even though the small intestine has a high cell turnover and large epithelial surface, adenocarcinomas develop much less frequently than in the large intestine. Several hypotheses have been postulated to explain this disparity in occurrence and these include: dilution of potential carcinogens due to the more liquid nature of small intestinal contents allows decreased mucosal contact; rapid transit time in the small intestine allows decreased mucosal contact of potential luminal carcinogens; presence of Paneth cells in the small intestine aids in antibacterial activity; lack of anaerobic bacteria in the small intestine that are able to convert bile salts to potential carcinogens; large amounts of lymphoid tissue (lamina propria and Peyers patches) that provide increased immunosurveillance against tumor cells; large amounts of mucosal enzymes that can detoxify luminal contents; less mechanical trauma to the mucosa due to more liquid luminal contents; and crypt stem cells are further away from contact with potential luminal carcinogens.(1)
Intestinal adenocarcinomas can often be further described based on their predominant morphologic appearance into acinar, papillary, mucinous, signet-ring cell, undifferentiated, or adenosquamous subtypes. In general, small intestinal adenocarcinomas are uncommon in domestic animals. However, in some countries, small intestinal adenocarcinoma can be common in cattle and is associated with ingestion of bracken fern and bovine papillomavirus type 4 infection.(2) Tumors are usually multiple and vary from adenoma to carcinoma affecting all levels of the small intestine. In sheep, there has also been an association with bracken fern ingestion and herbicide use.(2) Unlike in cattle, these tumors are usually mid-jejunal and solitary.
An additional finding in this marmoset was the presence of a pale eosinophilic amorphous material in widespread tissues (gastrointestinal tract, spleen, liver, kidney, adrenal gland, and thyroid gland). Congo red stain of a replicate section of kidney from this marmoset confirmed that the eosinophilic material is amyloid. Systemic AA or reactive secondary amyloidosis has been reported in common marmosets, usually related to a chronic inflammatory process that results in elevated serum amyloid A (SAA) protein levels. A genetic factor may also play a role in the common marmoset.(4) In addition to the small intestinal adenocarcinoma, this marmoset exhibited marked to severe granulomatous and eosinophilic cholangitis with intralesional degenerate parasitic remnants suggesting that this chronic inflammatory process likely contributed to elevated SAA levels with resulting widespread amyloid deposition.
Note: Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the U.S. Army.Â
Research involving this marmoset was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 2011. This research was conducted under an Institutional Animal Care and Use Committee (IACUC) approved protocol. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.Â
JPC Diagnosis:
1. Jejunum: Adenocarcinoma, mucinous type.
2. Jejunum, lamina propria: Amyloidosis, multifocal, moderate.Â
3. Jejunum: Ulcer, focal.Â
Conference Comment:
In addition to playing a role in the Wnt signaling pathway, β-catenin activity also has effects on cell-cell organization. β-catenin normally binds to the cytoplasmic domain of type I cadherins, facilitating linkage to the actin cytoskeleton and contributing to normal cellular structure and organization. Perturbed interactions between β-catenin and type I cadherins destabilize cell-cell interactions and thus promote the loss of cell cohesion which may contribute to metastatic spread of neoplastic cells.(7)
References:
2. Head KW, Cullen JM, Dubielzig RR, Else RW, Misdorp W, Patnaik AK, et al. In: Schulman Y, ed. Histological Classification of Tumors of the Alimentary System of Domestic Animals, 2nd series, Vol. X. Washington, DC: Armed Forces Institute of Pathology/ American Registry of Pathology; 2003:89-94.
3. Johnson LD, Ausman LM, Sehgal PK, King, Jr. NW. A prospective study of the epidemiology of colitis and colon cancer in cotton-top tamarins (Saguinus oedipus). Gastroenterology. 1996:110:102-115.
4. Ludlage E, Murphy CL, Davern SM, Solomon A, Weiss DT, Glenn-Smith D, et al. Systemic AA amyloidosis in the common marmoset. Vet Pathol. 2005;42(2):117-124.
5. Miller AD, Kramer JA, Lin KC, Knight H, Martinot A, Mansfield KG. Small intestinal adenocarcinoma in common marmosets (Callithrix jacchus). Vet Pathol. 2010;47(5):969-976.
6. Riddell RH, Petras RE, Williams GT, Sobin LH. In: Rosai J, ed. Atlas of Tumor Pathology: Tumors of the Intestines, 3rd series, Fascicle 32. Washington, DC. Armed Forces Institute of Pathology/ American Registry of Pathology. 2002:189-194.
7. Turner JR. The gastrointestinal tract. In: Kumar V, Abbas AK, Fausto N, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Elsevier/Saunders; 2010:822-825.
8. Valverde CR, Tarara RP, Griffey SM, Roberts JA. Spontaneous intestinal adenocarcinoma in geriatric macaques (Macaca sp.). Comp Med. 2000;50(5):540-544.