Wednesday Slide Conference, 2025-2026, Conference 1, Case 3
Signalment:
10 yr Gypsy Vanner mare, Equus caballus, horse.History:
Tissue from a 10-year-old Gypsy Vanner mare with a 24-hour history of colic. On presentation, the horse was tachycardic with decreased borborygmi in all quadrants. An abdominal ultrasound was performed that identified increased motility on the right side, decreased motility on the left side, and severely thickened sections of intestine. An abdominocentesis found increased lactate (4.0 mmol/L). Peripheral lactate was in the normal range (1.5 mmol/L). The mare was taken into surgery where a focal area of thickened small colon was identified, and a resection and anastomosis was performed along with biopsy of the affected site. Ultimately the mare did poorly following surgery, and was euthanized; a postmortem examination was not performed, and examination was limited to biopsy tissues removed during surgery.Gross Pathology:
The right frontal lobe was slightly enlarged (asymmetry). On cut surface, the white matter was mildy expanded and yellow (edema). A 1.1x0.5 cm, soft, red-brown area was within the right thalamus (malacia).Laboratory Results:
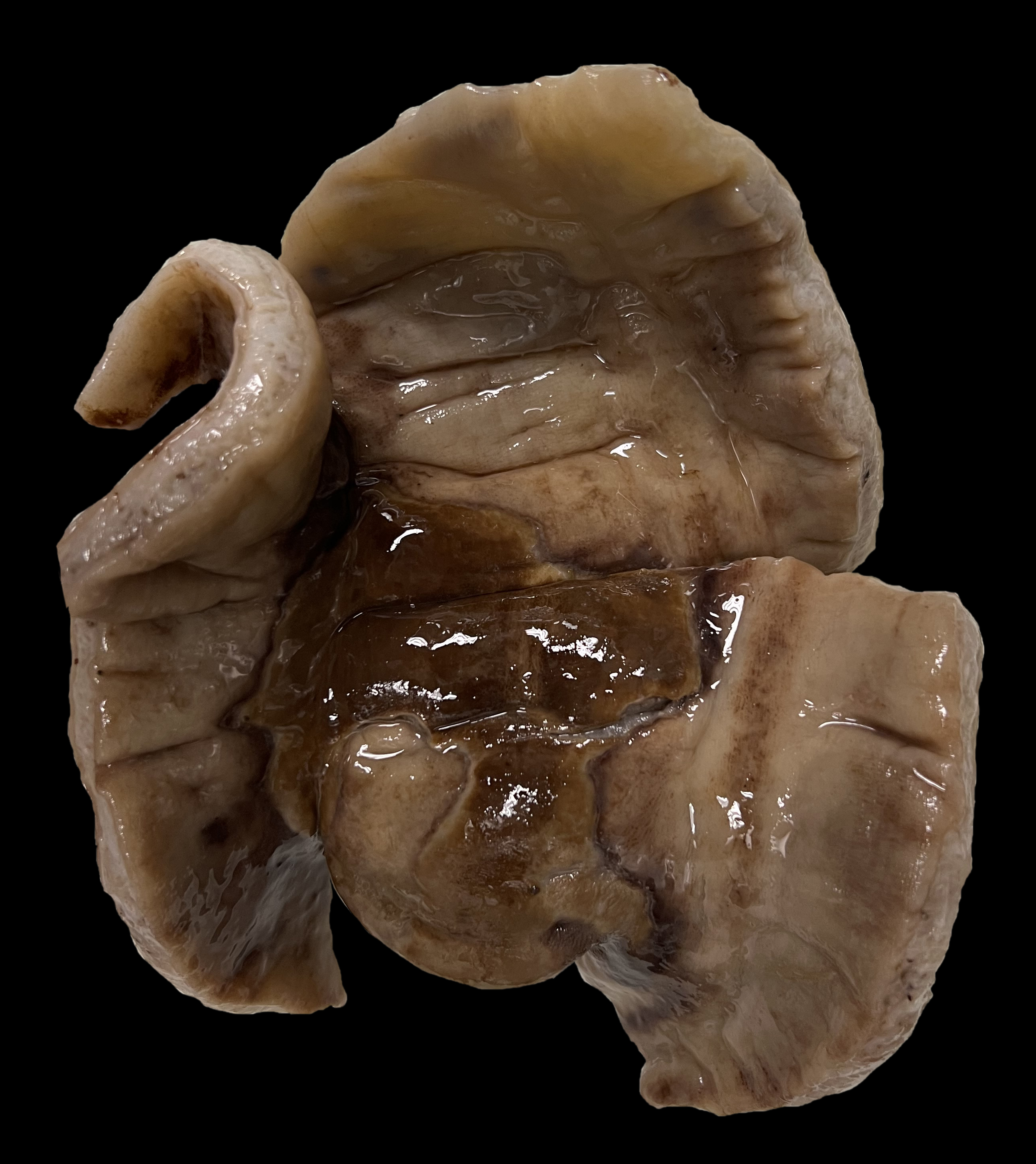
A segment of opened small colon measuring 16cm in length and 17cm in width was submitted for histologic evaluation. The segment is 1cm thick on the margins, narrowing to 0.4cm thick in the center of the section. On the mucosal surface, there is a focal, well demarcated, irregularly marginated tan to brown plaque rimmed in dark red that measures 8x9cm. Peripheral lactate: 1.5 mmol/L Abdominal effusion lactate: 4.0 mmol/LMicroscopic Description:
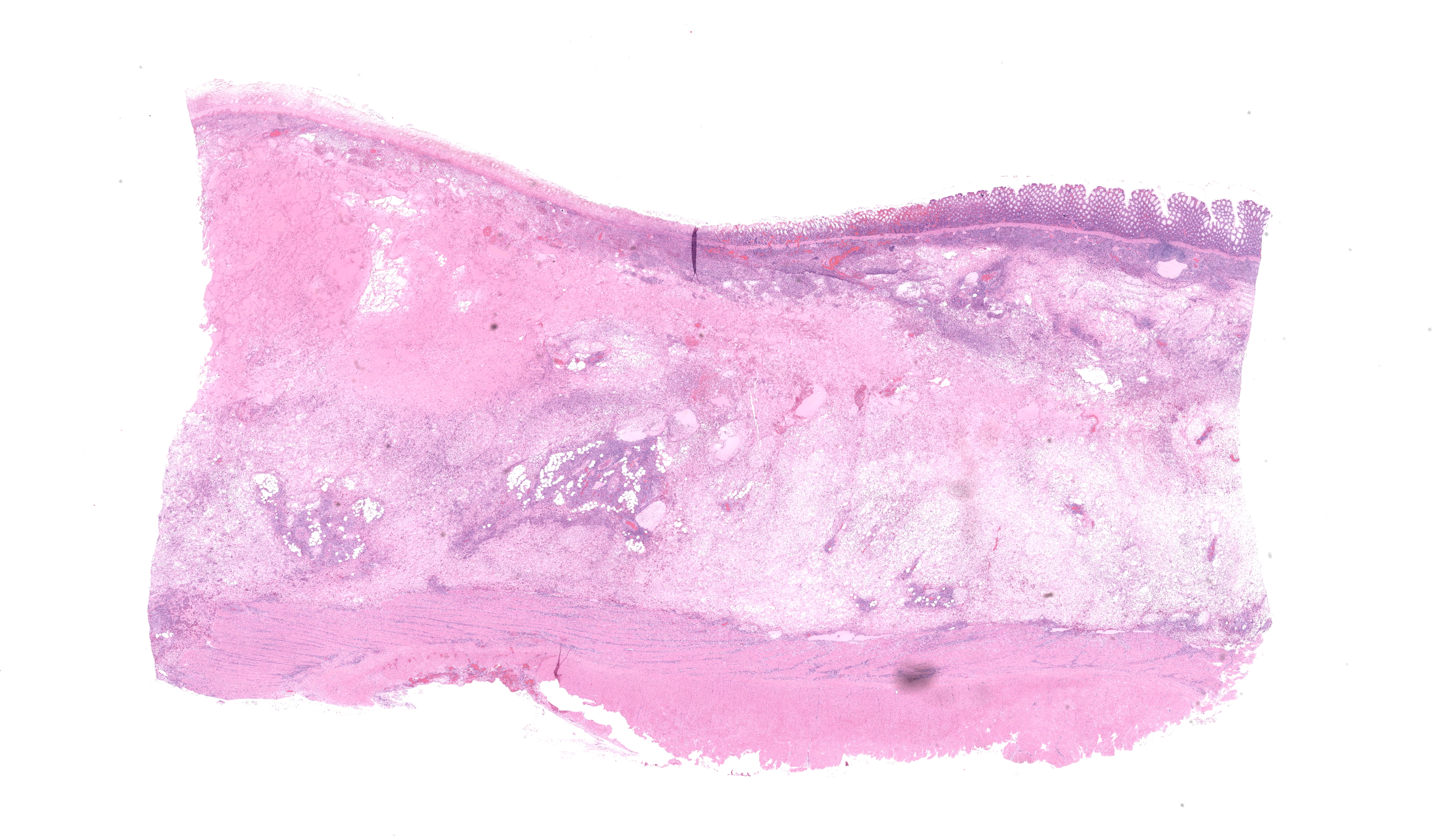
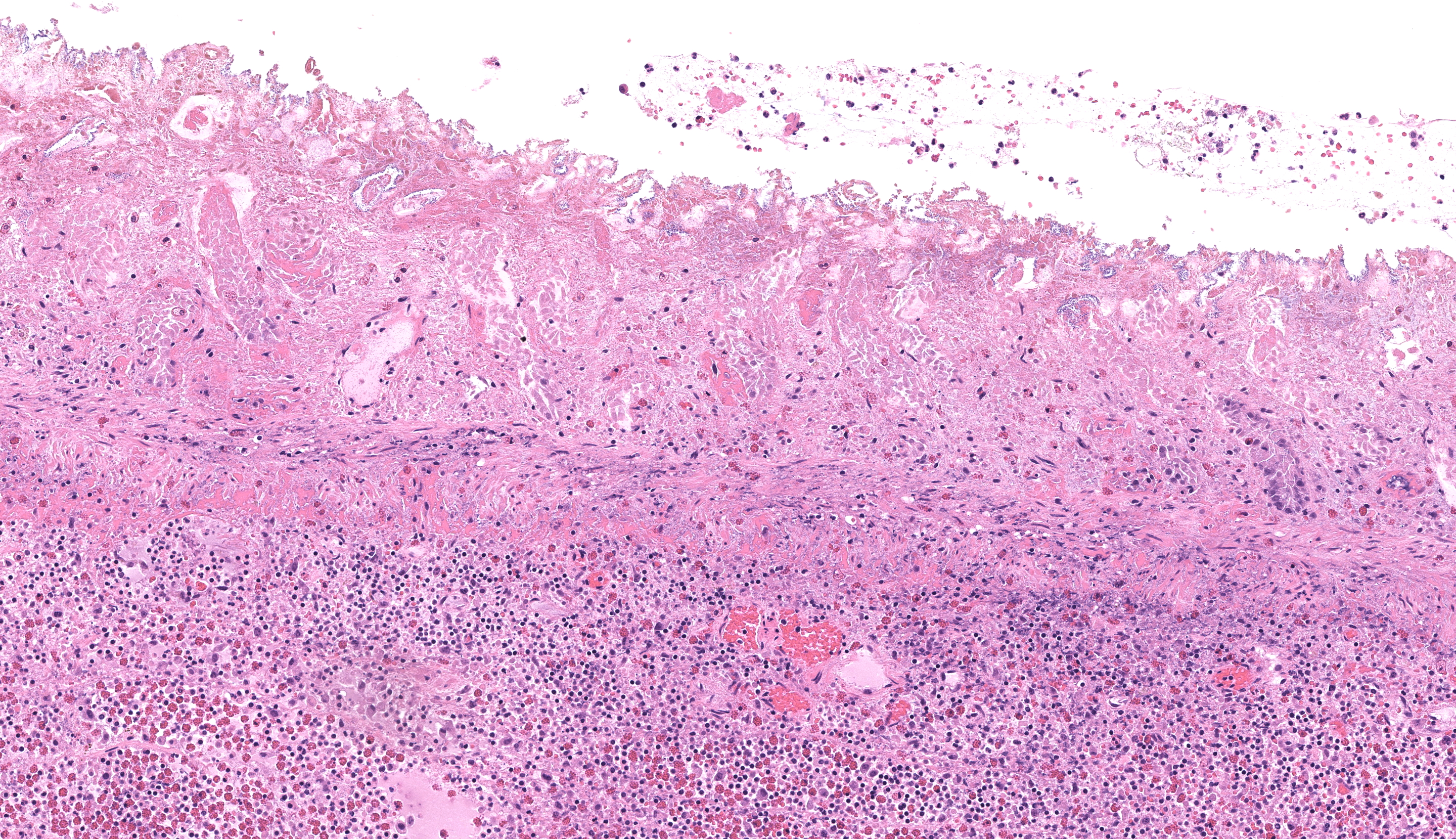
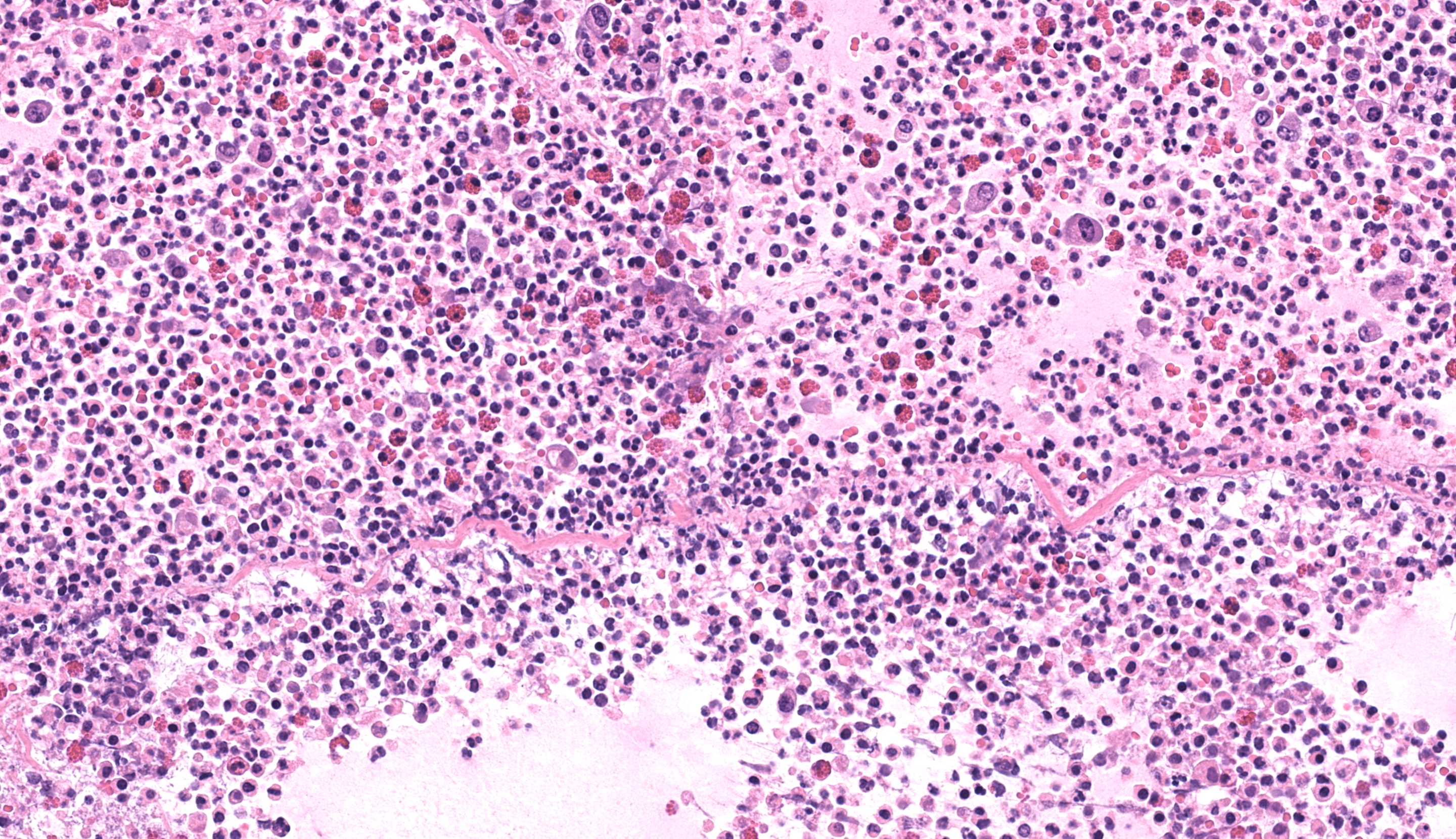
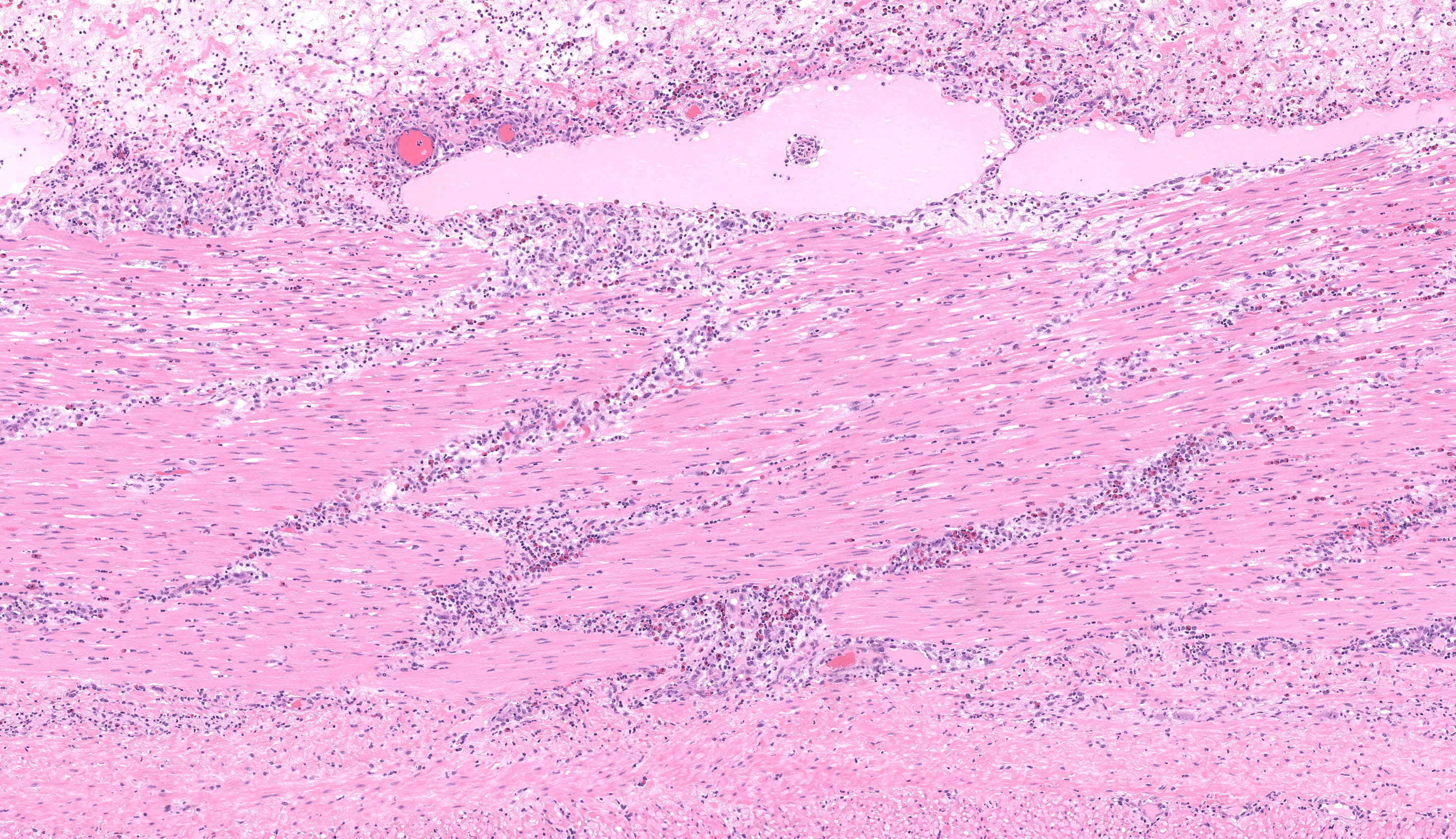
Small colon: Markedly, transmurally expanding the bowel wall, with the submucosa and muscularis layers most prominently affected, are numerous eosinophils, macrophages, and fewer lymphocytes, plasma cells, and neutrophils. The mucosa is mildly expanded by described inflammatory infiltrates, and vessels within the mucosa are congested with multifocal, variably minimal to moderate numbers of extravasated red blood cells (hemorrhage). Segmentally, approximately 50% of the mucosa has relatively sharply-demarcated loss of differential staining with variable retention of cellular architecture (coagulative necrosis) intermixed with foci comprised of karyorrhectic nuclear debris and granular eosinophilic material (lytic necrosis). Necrosis extends regionally into the superficial underlying submucosa. Beyond described inflammatory cells, the submucosa is further severely expanded by abundant homogenous pale eosinophilic fluid (edema), resulting in the submucosa comprising approximately 70% of the entire thickness of the bowel wall. Within the tunica muscularis, described inflammatory cells infiltrate between and separate branching bundles of smooth muscle, often loosely associated with small caliber vessels lined by hypertrophied endothelial cells. Within both the submucosa and muscularis, inflammatory cells are multifocally accompanied by mild proliferation of plump spindle cells (fibroblasts). The serosa is mildly affected by inflammation with associated multifocal vascular congestion and patchy hemorrhage. Grocott methenamine silver (GMS) stain is performed on tissues, and no etiologic agents are identified.Contributor's Morphologic Diagnoses:
Small Colon: Marked, multifocal to coalescing, subacute, submucosal and tunica muscularis-focused, eosinophilic, histiocytic, and neutrophilic colitis with marked diffuse edema and regional acute necrosis.Contributor's Comment:
The key histologic findings in this lesion are 1) the marked transmural infiltration of eosinophils within the segment of thickened bowel and 2) the particular concentration of eosinophils within the submucosa and muscularis of the affected bowel. In the horse, scattered eosinophils are commonly found within normal tissues of the gastrointestinal tract, but recruitment of eosinophils in diseased states are less common and most frequently attributed to parasitic migration, pythiosis, type I hypersensitivity, or idiopathic inflammatory conditions10,17. The eosinophilic inflammatory conditions with unknown causes have been categorized as part of an inflammatory bowel disease (IBD) complex14. Two distinct idiopathic causes will be discussed in detail: idiopathic focal eosinophilic enteritis (IFEE) and chronic eosinophilic enteritis (part of the multisystemic epitheliotropic eosinophilic disease, MEED). Idiopathic eosinophilic enterocolitis, or diffuse eosinophilic enteritis as proposed by Makinen et al., is a third idiopathic condition grouped into the IBD complex that is localized to the alimentary tract and consists of diffuse infiltrates of eosinophils with fewer macrophages than IFEE lesions and no obstructive disease, but with an otherwise similar histologic appearance2,9,14,17.IFEE was first identified in the early 1990s and since then has been documented in the United Kingdom, Republic of Ireland, United States and South Africa1,12. Archer et al. found IFEE to be an emerging disease with an increased incidence of cases from 2002 to 2010 in addition to possible seasonal and geographic correlations when cases are compared to control colic patients1. Clinical signs consist of acute colic, and focal circumferential thickening of the intestines can be identified on rectal palpation or by ultrasound1. The focal lesions are most often identified within the small intestine but have also been noted in the dorsal left colon and can result in obstructive disease4,12,16,17. Surgical resection of the affected segments of bowel is thought to be curative1,2. Histologically, the lesions consist of focal infiltrations of eosinophils and macrophages within the submucosa and tunica muscularis with increased numbers of mononuclear cells2,9,17. The lamina propria is typically expanded with fewer eosinophils and abundant lymphocytes, plasma cells, and macrophages2,9,17. A causative agent has not been identified, but exposure to stagnant water has been a proposed contributor1. A type I hypersensitivity or parasitism cannot be ruled out given the nature of the disease.
Chronic eosinophilic enteritis has been documented in conjunction with MEED since the early 1980's11,19. Young horses are most at risk for developing the disease14,18. Clinical symptoms typically develop over a period of months with eosinophilic and granulomatous lesions noted most often in the skin, biliary ducts, pancreas, lung, salivary glands, and mesenteric lymph nodes15,17. Clinical presentation often consists of severe weight loss that develops over months and pitting edema attributed to enteric loss of plasma protein from chronic inflammation3. With colonic involvement, horses will develop diarrhea17. The intestinal histologic lesions consist of mucosaI, submucosal, and often transmural eosinophilic infiltrates accompanied by mast cells, macrophages, lymphocytes, and plasma cells3,17,18. In the author's experience, this will often include formation of discrete eosinophilic granulomas, which is in contrast to IFEE. Often lesions will contain extensive fibrosis, villous atrophy, and muscularis mucosa hypertrophy17. Proposed pathogeneses include a type I hypersensitivity to migration of enteric parasite larva or ingested allergens, or undetected IL-5 secreting T-cell lymphoma as supported by a documented case of CD3+ intestinal lymphosarcoma with concurrent MEED in the horse8,17.
In other species, eosinophilic enterocolitis entities attributed to a variety of causes has been identified in humans, dogs, cats, ferrets, and cattle either alone or with multisystemic inflammation5,6,7,13.
Collectively, while occurring in an unreported location (small colon), the clinical presentation, gross finding of focal thickening of the bowel, and histologic appearance of this case support a diagnosis of IFEE. Histologically, there is extensive submucosal and tunica muscularis infiltration by predominantly eosinophils and macrophages, with fewer numbers of neutrophils, lymphocytes, plasma cells. While neutrophils are not commonly described in IFEE lesions, we attribute the presence of neutrophils to regional necrosis of the bowel, which we suspect is secondary to obstruction. The concentration of inflammatory cells within the submucosa and muscularis is reportedly a key feature of IFEE, supporting this diagnosis. The differential diagnosis of chronic eosinophilic enteritis as a component of MEED is not favored in this case due to the absence of eosinophilic granulomas, lesion distribution in the bowel wall, and absence of other known affected systems, although lack of postmortem examination precludes true evaluation of the latter point. Lastly, idiopathic eosinophilic enterocolitis/diffuse eosinophilic enteritis is not favored in this case due to the focal and obstructive nature of the lesion, as well as the abundance of macrophages. Despite our preference for this lesion representing IFEE, we recognize that these conditions are not extensively characterized within veterinary pathology literature, so we cannot fully rule out that this lesion may reflect a variant of one of these other idiopathic conditions. In this case, infectious agents (i.e. fungal or fungal-like organisms, e.g. Pythium spp.) were not identified via H&E and GMS evaluation, but we cannot rule out that undetected agents may be inciting this lesion. Overall, this case highlights the need for further investigation and characterization of equine idiopathic eosinophilic inflammatory bowel conditions.
Contributing Institution:
The Ohio State University College of Veterinary Medicine, Department of Veterinary Biosciences ref="https://vet.osu.edu/biosciences">https://vet.osu.edu/biosciencesJPC Diagnoses:
Colon: Colitis, eosinophilic, subacute, focally extensive and transmural, severe, with segmental necrosis and marked edema.JPC Comment:
The contributor provided a thorough comment comparing two entities that share many similarities yet have some distinct differences. Conference discussion was largely centered around comparing idiopathic focal eosinophilic enteritis (IFEE) and multisystemic epitheliotropic eosinophilic disease (MEED), with the clearest differences being the distribution of lesions and histologic presence of eosinophilic granulomas in MEED. However, it was also stated that the lack of eosinophilic granulomas does not rule out MEED and attention should be given more so to distribution and clinical history due to the similarities in these conditions. There was also special attention given to the segmental coagulative necrosis in this case, which conference participants largely attributed to the histologically evident vasculitis. Ultimately, in light of the clinical and histologic findings in this case, conference participants agreed with the contributor?s diagnosis of IFEE. Grocott's methenamine silver (GMS) and periodic acid-Schiff (PAS) stains were run on this case and did not reveal any infectious agents, which is consistent with what the contributor reported in their own work-up.References:
- Archer DC, Costain DA, Sherlock C. Idiopathic focal eosinophilic enteritis (IFEE), an emerging cause of abdominal pain in horses: the effect of age, time and geographic location on risk. PloS One. 2014;9(12):e112072.
- Archer DC, Edwards GB, Kelly OF, et al. Obstruction of equine small intestine associated with focal idiopathic eosinophilic enteritis: An emerging disease? Vet J. 2006;171 :504-512.
- Bosseler L, Verryken K, Bauwens C, et al. Equine multisystemic eosinophilic epithelotropic disease: a case report and review of literature. NZ Vet J. 2013;61 :177-182.
- Edwards GB, Kelly DF, Proud man CJ. Segmental eosinophilic colitis: a review of 22 cases. Equine Vet J. 2000;32:86-93.
- Fox JG, Palley LS, Rose R. Eosinophilic Gastroenteritis with Splendor-Hoeppli Material in the Ferret. Vet Pathol. 1992;29:21-26.
- Fushimi Y, Takagi M, Kawaguchi H, et al. Three cases of idiopathic eosinophilic enteritis with chronic obstinate diarrhea in Japanese Black fattening cattle. J Vet Med Sci. 2015;77(3):337-340.
- Hendrick M. A spectrum of hypereosinophilic syndromes exemplified by six cats with eosinophilic enteritis. Vet Pathol. 1981 ;18:188-200.
- La Perle KM, Piercy RJ, Long JF, et al. Multisystemic, eosinophilic, epitheliotropic disease with intestinal lymphosarcoma in a horse. Vet Pathol. 1998;35(2):144-6.
- Makinen P, Archer DC, Baptiste KE, et al. Characterization of the inflammatory reaction in equine idiopathic focal eosinophilic enteritis and diffuse eosinophilic enteritis. Equine Vet J. 2008;40:386-392.
- Morton LD, Morton DG, Baker GJ, et al. Chronic eosinophilic enteritis attributed to Pythium sp. in a horse. Vet Pathol. 1991;28:542-544.
- Pass DA, Bolton JR. Chronic Eosinophilic Gastroenteritis in the Horse. Vet Pathol. 1982;19:486-496.
- Piketh G, Henning A, Smit Y. Idiopathic focal eosinophilic enteritis associated with ileocaecal and ileal obstruction in a 10-year-old warmblood gelding. Vet Rec. 2022;1 0:e421.
- Quigley PJ, Henry K. Eosinophilic enteritis in the dog: A case report with a brief Review of the Literature. J Comp Path. 1981 ;91 :387-392.
- Schumacher J, Edwards JF, Cohen ND. Chronic idiopathic inflammatory bowel diseases of the horse. J Vet Intern Med. 2000;14(3):258-65.
- Singh K, Holbrook TC, Gilliam LL, et al. Severe pulmonary disease due to multisystemic eosinophilic epitheliotropic disease in a horse. Vet Pathol. 2006;43: 189-193.
- Swain JM, Licka T, Rh ind SM, et al. Multifocally eosinophilic enteritis associated with a small intestinal obstruction in a standard bred horse. Vet Rec. 2003;152:648-651.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. Vol 2. 6th ed.St. Louis, MO: Elsevier Saunders; 2015:96.
- Villagran CC, Vogt D, Gupta A, et al. Inflammatory bowel disease characterized by multisystemic eosinophilic epitheliotropic disease (MEED) in a horse in Saskatchewan, Canada. Can VetJ. 2021;62:1190-1194.
- Wilkie JSN, Yager JA, Nation PN, et al. Chronic eosinophilic dermatitis: a manifestation of a multisystemic, eosinophilic, epitheliotropic disease in five horses. Vet Pathol. 1985;22:297-305.