Signalment:
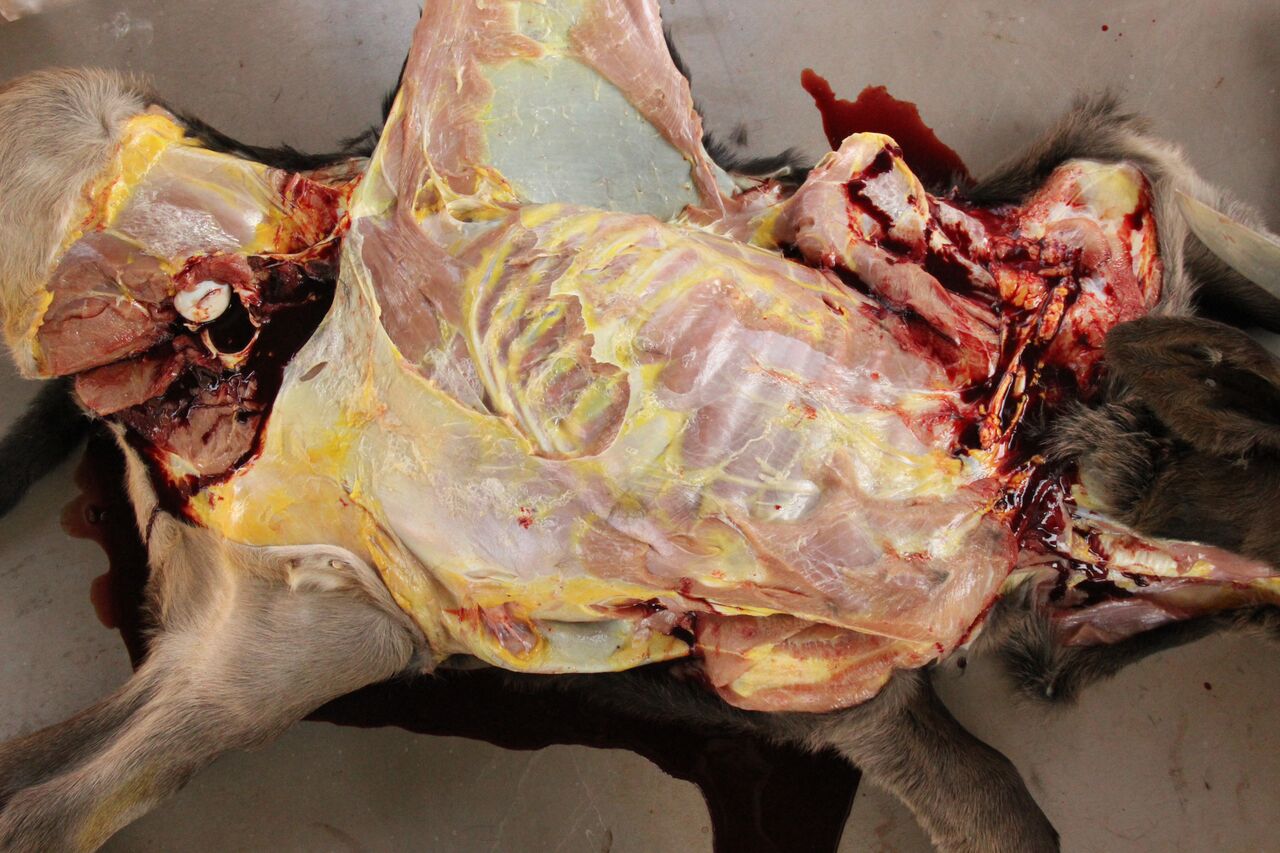
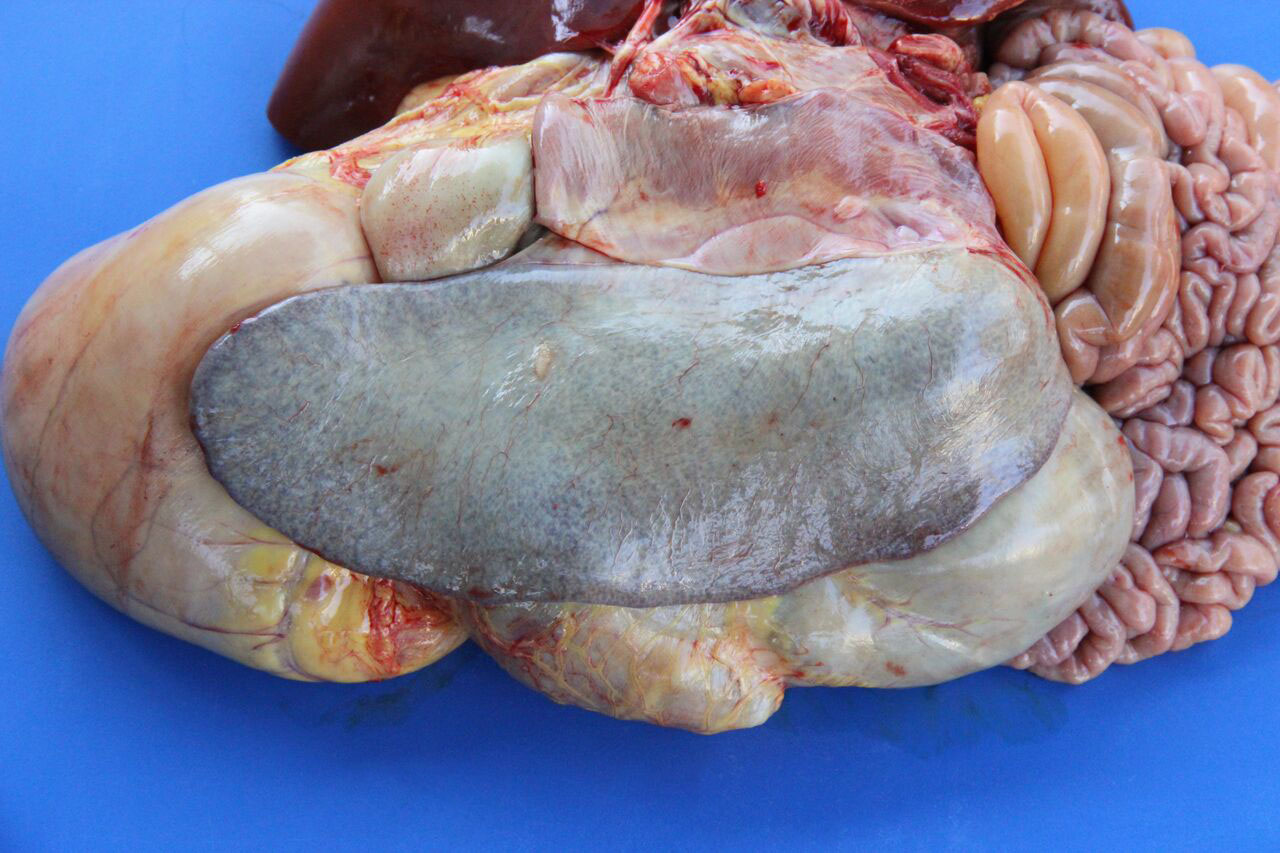
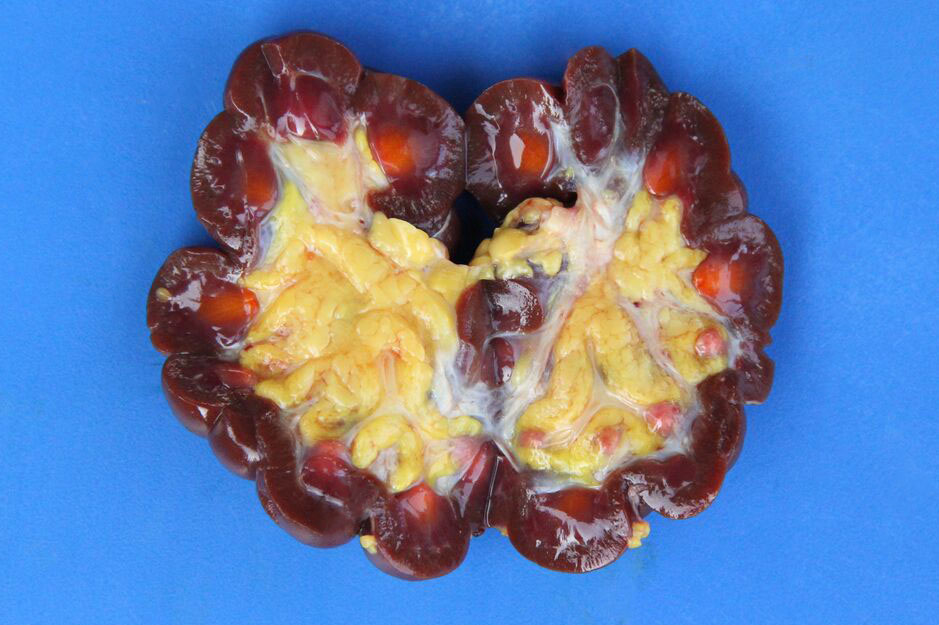
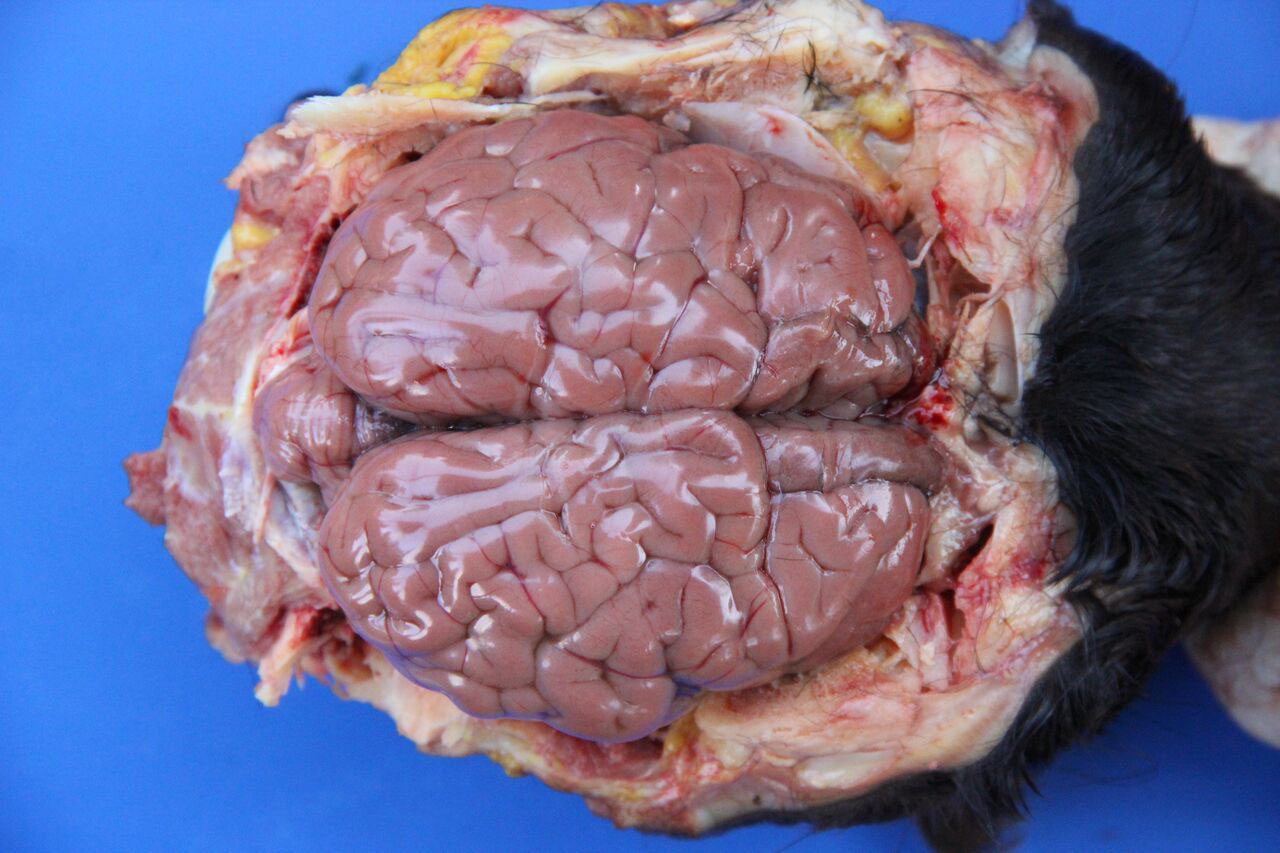
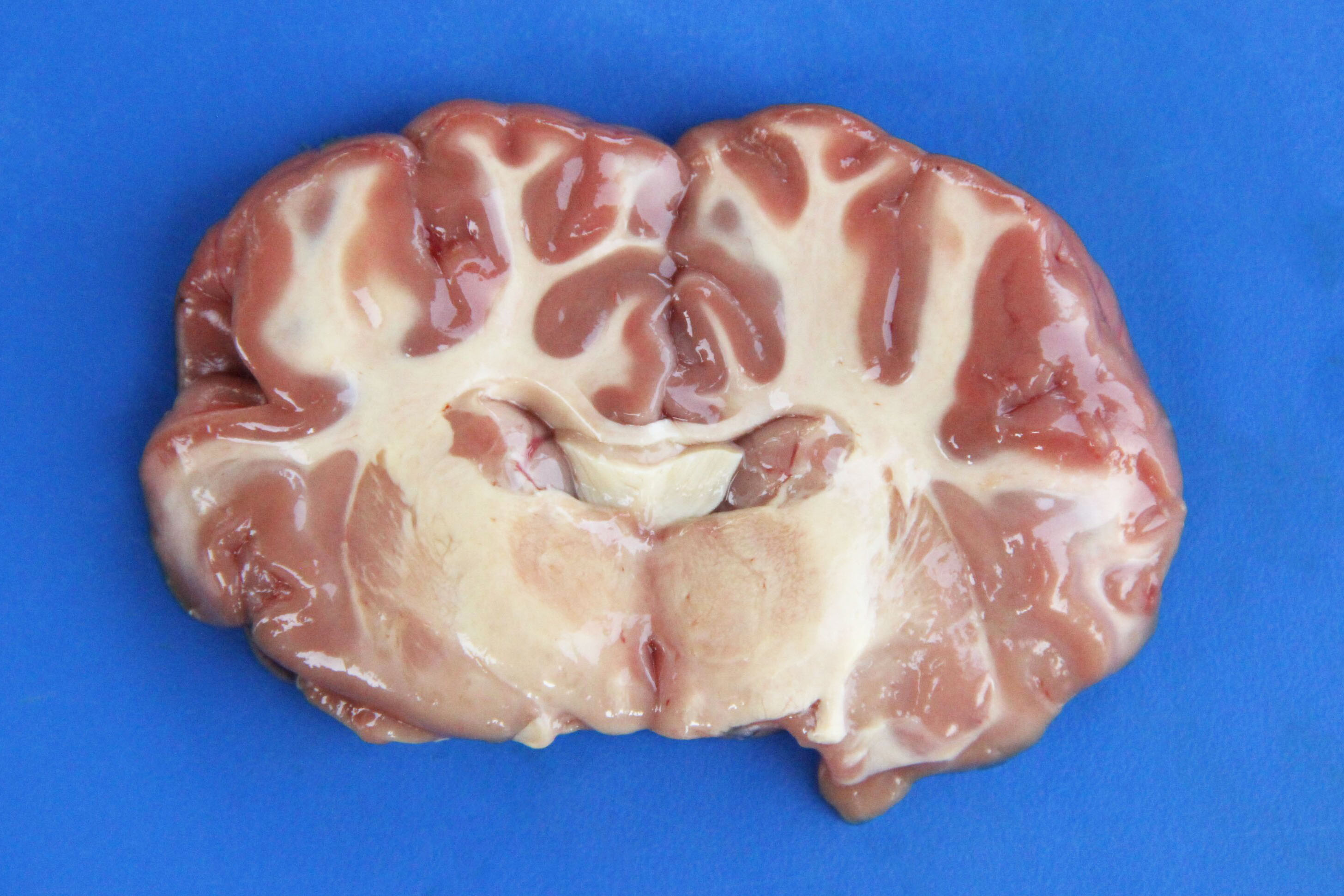
Gross Description:
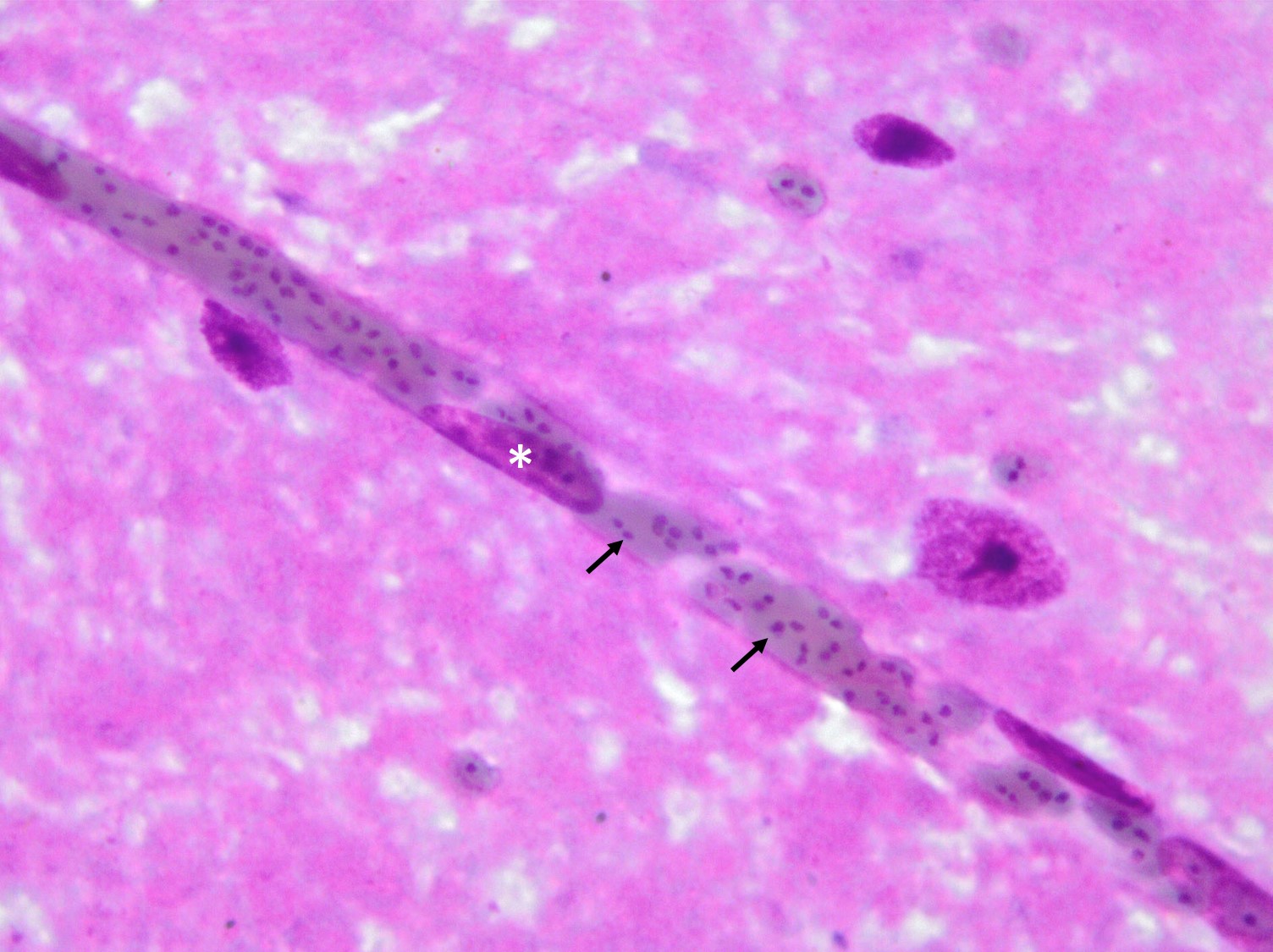
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
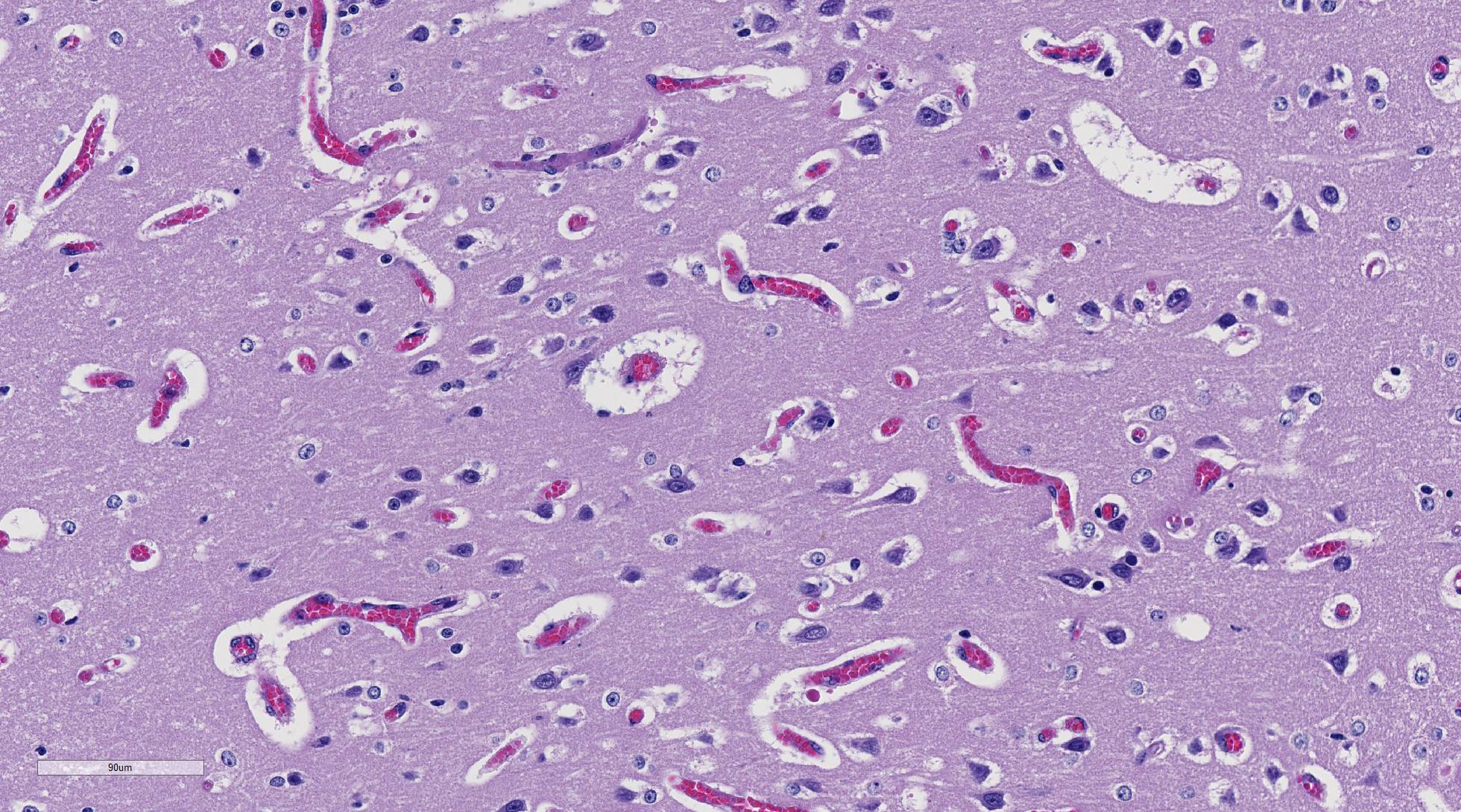
In Romanowsky-stained squashes from telencephalic cortex, capillaries appeared clogged with intraerythrocytic small (2 μm-diameter) paired or single spherical basophilic organisms (morphology compatible with Babesia bovis). Similar organisms were also observed within erythrocytes from blood smears.
Condition:
Contributor Comment:
Babesia was first described by Babés in Romania as a parasite of bovine erythrocytes. Although it is possible for a single Babesia species to infect more than one vertebrate host (e.g., B. microti affects rodent and humans; B. divergens and B. bovis affect cattle and humans), Babesia spp. are typically host specific.2
Bovine babesiosis (aka, piroplasmosis, Texas fever, redwater, and tick fever) is a febrile hemolytic condition caused by one of at least seven species the protozoan organisms Babesia spp. It is characterized by extensive intravascular hemolysis leading to depression, anemia, icterus, hemo-globinuria, and, in the case of B. bovis infections, neurological signs.2
In Brazil, bovine babesiosis is caused by B. bovis (formerly B. argentina) and/or B. bigemina and is transmitted to cattle by the tick Rhipicephalus (Boophilus) microplus.8,10 The disease is frequently, but not always associated with icterus and hemoglobinuria and the animal may become extremely ill before severe anemia, parasitemia or hemoglobinuria are apparent.5,15
In general, the disease distribution follows that of the vector ticks producing three distinct epidemiological situations. The disease does not occur in areas without the tick vector. In areas of enzootic instability, there is an alternation of warm and cold seasons. The cold period prolongs the free-living stages of the tick, allowing cattle extended periods without vector contact, resulting in a significant drop in antibodies due to the absence of Babesia infection. When the warm period returns, the tick parasite load increases and outbreaks occur. In enzootic areas, weather condition allow the presence of tick on cattle all year round, which confers high level of lasting immunological protection.1
Factors influencing the occurrence of babesiosis outbreak include (1) over infestation by vector ticks resulting in a high inoculum of Babesia: (2) long periods without ticks with resultant loss of immunity, and (3) stress factor and nutritional deficiencies which can induce a drop in immunity and vulnerability to the disease.2 Calves are typically more typically more resistant to infection by Babesia sp. than adult cattle.15 Mortality is lower in enzootic areas due to resistance to infection.
B. bovis is small, pleomorphic apicomplexan parasite, and can occur as single or as paired pear-shaped bodies joined at an obtuse angle within the center of the mature erythrocyte. The round forms measure 1-1.5 µm, and the pear-shaped bodies 1.5 x 2.4 µm in size. Vacuolated signet ring forms are particularly common. Hosts are cattle, buffalo, and deer.14
The incubation period for bovine babesiosis is typically 2-3 weeks1,15 for the natural disease. The natural infection caused by B. bovis tends to present a longer incubation time than that caused by B. bigemina. Cases of extremely short incubation periods (seven days) have been reported for B. bovis.13,14
Infection occurs after the tick vector feeds on the host. After the inoculation of the infectious sporozoites, the parasite penetrates the erythrocytes in the definitive host where they form a parasitophorous vacuole and change to the trophozoite form. Later these trophozoites undergo binary division, usually forming two merozoites. Ticks acquire Babesia infection during feeding on infected animals.2
Affected cattle develop depression, anorexia, paleness of mucous membranes, and fever (40 degrees/C-42 degrees C). Icterus and hemoglobinuria are also common clinical signs, but they can be minimal or absent in cases of peracute or acute disease. The elevation of serum activity of AST and GGT, observed in this case, may be due to the hepatic centrilobular hypoxic necrosis.1 Additionally, this calf had omphalitis, which probably resulted in septicemia (high fibrinogen) which would explain the low blood sugar.4
B. bovis causes the most severe form of babesiosis in cattle in which peripheral circulatory disturbances with sequestration of parasitized erythrocytes in the peripheral circulation are unique features.3,11,12,16 In southern Brazil this form of disease is found in approximately two-thirds of the cases of babesiosis caused by B. bovis and is virtually always fatal.11
Necropsy findings include yellow discoloration (icterus) of mucosae, subcutaneous tissue, muscle fasciae and the intimal surface of arteries. In acute or peracute cases (which include the cases of cerebral babesiosis) icterus can be mild or absent. The serosal membranes of the abdominal viscera have hemoglobin imbibition. The liver is swollen, with rounded edges, and is yellow or tan discolored. The biliary vesicle is usually markedly distended by dark-green inspissated bile. Subepicardial and subendocardial hemorrhages (petechiae and ecchymosis) are virtually always observed. In cases where hemoglobinuria is a prominent sign, the kidneys are diffusely dark red-brown (hemoglobinuric nephrosis) and the urine is dark-red (red water disease). There is always some degree of splenomegaly. In severely enlarged spleens the red pulp prolapses on the cut surface. In cerebral babesiosis, the grey matter of the telencephalic, and cerebellar cortex and that of the basal nuclei has a characteristic cherry pink color. Squashes made from cortical brain, stained with Wright-Giemsa reveal numerous capillaries engorged with parasitized erythrocytes.3,5,6
The encephalic lesion is characteristic of B. bovis infection and does not occur in any other Babesia sp. infection of cattle. However, it can be compared with the brain lesions seen in severe cases of malaria caused by Plasmodium falciparum. Microscopically the brain lesions are characterized by cortical capillaries diffusely engorged with red blood cells, and perivascular and perineuronal edema. In tissue sections of the brain, Giemsa and even HE stains demonstrate the parasites as small paired or single spherical faint basophilic bodies.1,15 Parasitized erythrocytes may also be seen in vessels of virtually all tissues, such as the interstitial capillaries in the kidney, heart, and in skeletal muscle.15 Other changes characteristic of hemolytic anemia are observed such hemoglobinuric nephrosis, centrilobular hepatic necrosis (due to hypoxia) and bile stasis.15
Ultrastructurally, capillaries in the brain are dilated and filled with densely packed parasitized erythrocytes. These erythrocytes have scalloped edges with fine strands apparently connecting adjacent red blood, as well as connecting erythrocytes and endothelial cells.5,6 Masses of lysed red blood cells which have undergone lysis but still contain intact parasites are frequently seen in capillaries. Changes in the capillary endothelium of the affected parts in the brain range from swelling of the cytoplasm and nucleus to necrosis. Perivascular and perineuronal spaces are enlarged. In the kidneys, capillaries are not packed as tightly with red blood cells as in the brain, and the parasitemia does not exceed 50% of red blood cells. Other changes are similar to those seen in the brain. Capillaries in the lung are packed with red blood cells, but only a small proportion of the cells are parasitized.5
Although usually reported resistant to babesiosis, calves in enzootic areas can be parasitized by R. microplus on the first day of life.8 Since the protection of neonates calves is conferred by passive immunity through colostral ingestion, a failure in the transfer of passive immunity from the dam can explain very young calves being affected by the disease. A lack of passive immunity caused by the failure of colostral ingestion in the calf of this report could be suspected since omphalitis was also present.
The pathogenesis of the disease caused by B. bovis in cattle is not completely understood, but some mechanisms are proposed.5,15 Acute disease is characterized by a hypotensive shock syndrome with vascular stasis and accumulation of parasitized erythrocytes in the peripheral circulation. It is accompanied by activation of coagulation/complement cascades and the release of vasoactive compounds resulting in vasodilation and circulatory stasis as well as generalized organ damage due to anoxia and toxic products from both parasites and damaged host tissue.
Parasite proteases cause hydrolysis of fibrinogen which results in the accumulation of large quantities of soluble fibrin complexes which are not cross-linked, as well as in altered fibrinogen in the circulation. Thus, the coagulability and viscosity of blood increases but insoluble fibrin is not produced, suggesting that classic disseminated intravascular coagulo-pathy is not a feature of B. bovis infections.5 The cause of cytoadherence of in B. bovis organisms to endothelial cells is also uncertain,5,15 but the Babesia organisms remodel the erythrocyte surface with their erythrocyte surface antigen proteins, which changes the membrane mechanical and adhesive properties.15 In the severe form of malaria caused by Plasmodium falciparum, a disease that in many aspects resemble cerebral babesiosis, the causative organism induce infected red cells to clump together and to stick to endothelial cells lining of small blood vessels (sequestration), which blocks blood flow. Several proteins, including P. falciparum erythrocyte membrane protein 1 (PfEMP1), associated and form knobs on the surface of erythrocytes. PfEMP1 binds to thrombospondin, VCAM-1, ICAM-1, CD36, and E-selectin on the endothelial cells. Erythrocytes sequestration decreases tissue perfusion and leads to ischemia, which is responsible for the manifestations of cerebral malaria, the major cause of death in children with malaria.9 Mild to minimal mononuclear perivascular cuffing can be observed in a few of the slides of this case. This change is of no clinical significance encountered in one-third of symptomless cattle.7
JPC Diagnosis:
Conference Comment:
The sequestration of infected erythrocytes within the microvasculature of the cerebrum and other visceral organs leads to occlusion of the vessels and subsequent hypoxic injury. Babesia bovis also releases vasoactive proteases that activate the hypotensive agent, kallikrein, which then activates another potent vasodilator, bradykinin.15 The dilatory effect of these vasoactive proteins shifts blood away from veins and further contributes to apparent anemia. Additionally, infected animals are typically markedly anemic secondary to both intravascular and extravascular hemolysis, described by the contributor.15 The combination of vascular congestion, vasodilation, and hemolysis leads to both metabolic alkalosis and a hemodynamic crisis, often resulting in the death of the animal.
Most conference participants noted congestion and dilation of the cerebral microvasculature in this case; however, some attendees offered a dissenting opinion that the apparent congestion is a result of post-mortem pooling of blood within the brain rather than an antemortem change. The conference moderator agreed with the majority of the participants that the cerebral congestion is part of the pathogenesis of this disease and likely represents a real lesion, rather than an artifact. Participants also astutely noted that while vessels are congested in both the grey and white matter histologically, congestion is only apparent within the grey matter macroscopically.15
References: