WSC 2023-2024, Conference 13, Case 4
Signalment:
Age and gender unknown, Sprague Dawley rat (Rattus norvegicus)
History:
The patient was enrolled in an IACUC protocol. Following surgery, the rat was not grooming, was urinating on itself, was very lethargic, and lost more than 15% of its body weight. There was no sign that it was eating food or gel packs. Euthanasia was performed and a necropsy was performed by the research technician.
Gross Pathology:
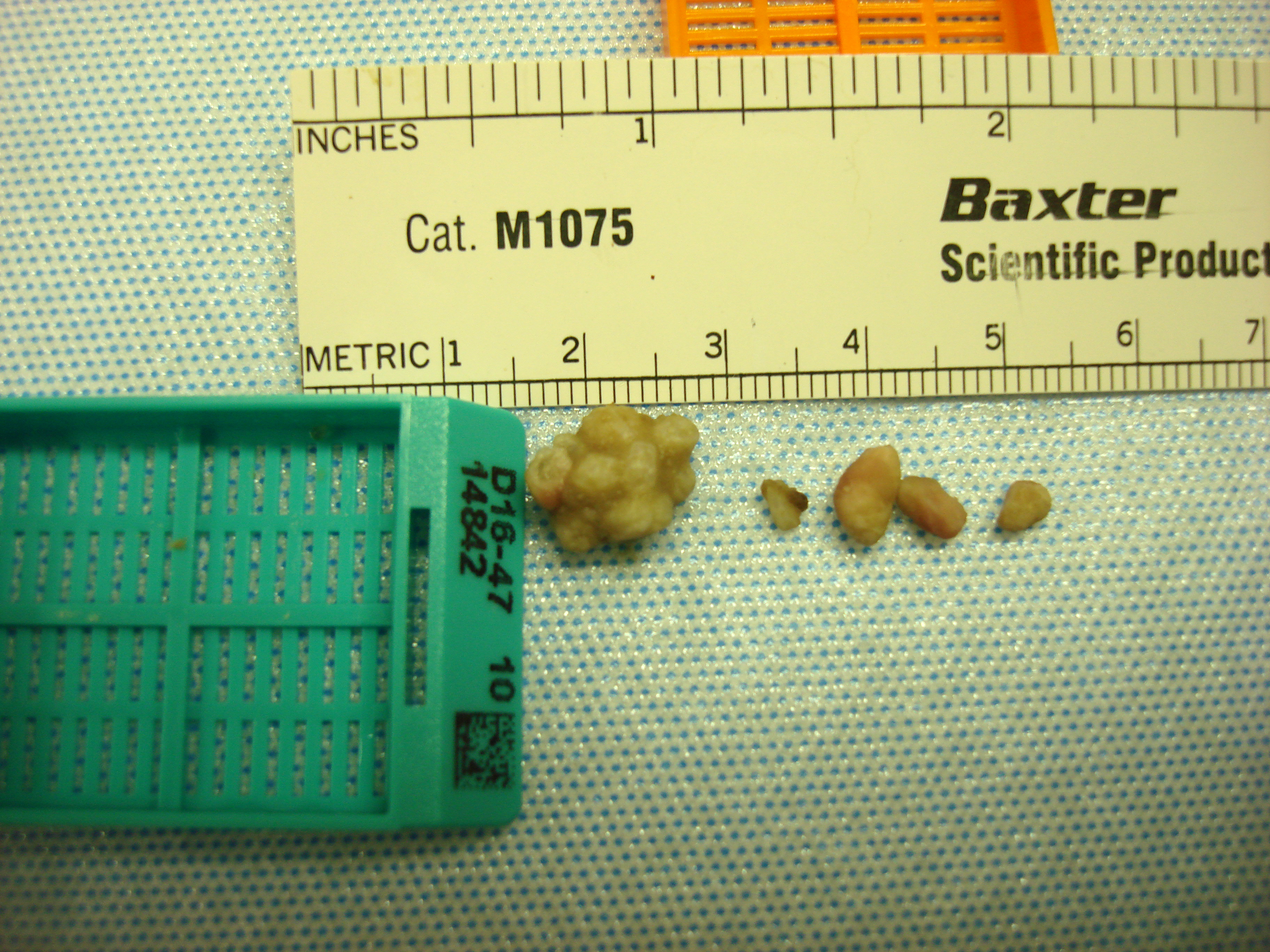
The bladder was distended and discolored. Urine collected aseptically was purulent and bloody. The stomach was full, but the rest of the gastrointestinal tract was empty. Both kidneys had multifocal white spots on the cortex. The remaining organs appeared normal. Gross dissection of the bladder revealed numerous uroliths.
Laboratory Results:
Proteus mirabilis was cultured from the urinary bladder. Stone analysis was not performed.
Microscopic Description:
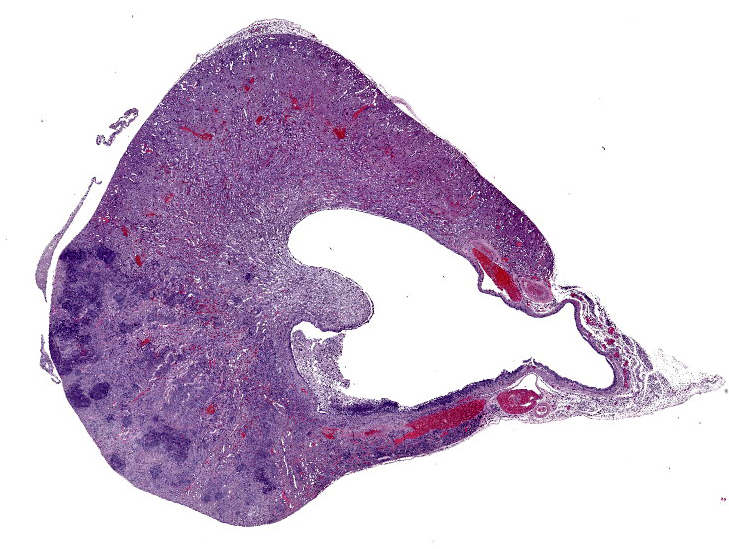
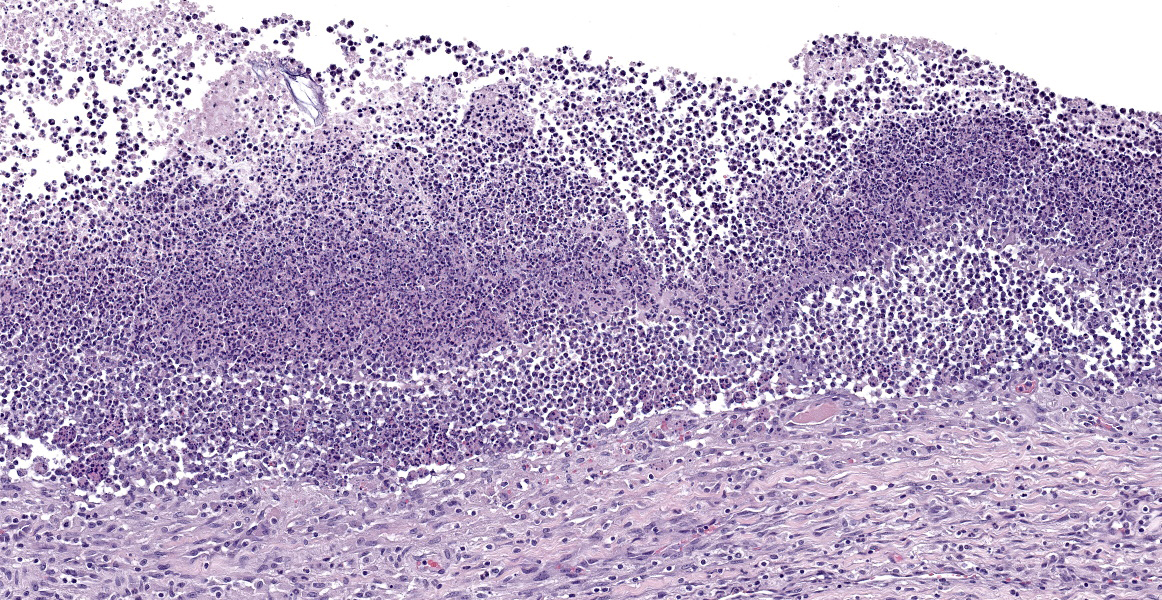
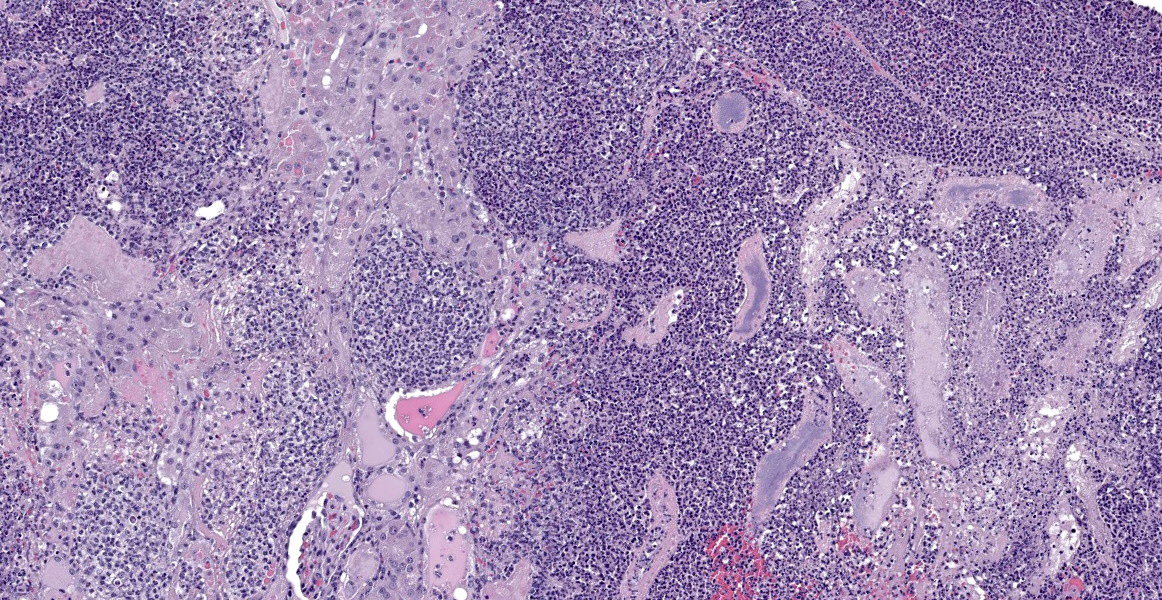
Affecting 40% of primarily the renal cortex, but extending into the medulla, tubules are necrotic and degenerate, lost and replaced, or expanded and widely separated by viable and degenerate neutrophils, necrotic cellular debris (lytic necrosis) and large colonies of basophilic bacterial rods. Tubules are degenerate or regenerating. Multifocally tubules have retention of cellular architecture and loss of differential staining (coagulative necrosis). Within tubules there is a homogenous eosinophilic material (proteinosis) or sloughed epithelium admixed with neutrophils and cellular debris (cellular cast) and rarely deeply basophilic crystalline material (mineral). The interstitium is expanded by the same cellular debris and fibrin, hemorrhage and edema. Glomerular changes include synechia, parietal cell hypertrophy, necrosis of mesangial cells and protein in Bowman’s space.
The renal surface is undulant with a mildly thickened capsule expanded by adherent fibrin and inflammatory cells. The renal pelvis is dilated (hydronephrosis) and contains previously described lytic necrosis and inflammatory cells which transmigrate the multifocally lost and necrotic (ulcerated) urothelium.
Contributor’s Morphologic Diagnosis:
Kidney: Pyelonephritis, suppurative, multifocal, severe, chronic, with large colonies of bacteria, tubular degeneration, regeneration and necrosis, protein and cellular casts, intratubular bacteria, hydronephrosis, and urethritis.
Contributor’s Comment:
Proteus mirabilis is a gram-negative, flagellated, swarming, commensal inhabitant of the intestinal tract in humans and the respiratory and digestive tract of mice.5 This bacteria may affect multiple organs by gaining access to the systemic circulation, inducing bacteremia, or can infect the urinary system through an ascending infection. In people, this is a common nosocomial cause of catheter associated urinary tract infections. In the dog and cat, Escherichia coli followed by Proteus mirabilis are the two most frequently isolated gram negative bacteria in urinary tract infections.3 On a more historic note, P. mirabilis has been a source of surgical infection via contaminated instrumentation and has rarely been implicated in cases of meningitis and cerebral abscesses in neonates.8
The pathogenesis of P. mirabilis involves its unique ability to differentiate from a swimmer cell to a swarmer cell depending on the environment. In a liquid environment, the bacteria swim, (called swimmers) while on a solid surface, such as a urinary catheter, they differentiate into swarmers forming rapidly moving rafts of bacteria.2 P. mirabilis has several virulence factors, including flagella for increased motility, adhesion factors for binding to epithelium in the urinary tract, and the ability to utilize host iron.1,2 This infection also creates an overwhelming neutrophilic response and damages host tissue via hemolysin, which is cytotoxic to epithelial cells, and urease. Urease breaks down urea to ammonia, raising the pH of the urine and precipitating the formation of stones which are often struvite or carbonate hydroxyapatite, thus causing additional mechanical epithelial damage.1,2
One current area of research has been the investigation of P. mirabilis and its potential to alter tumor hypoxia thereby inhibiting tumor growth.9 Through various mechanisms, such as gene delivery and immunomodulator delivery, Salmonella typhimurium, E. coli and Clostridium sp. have also been investigated for their potential roll in treating cancer.7 One particular study found that P. mirabilis may be a potential method for suppressing growth of primary breast cancer in murine models.9
Although not a common pathologic entity in veterinary medicine, this bacteria should be considered as a differential for cases of suppuarative pyelonephritis in rodents.
Contributing Institution:
Tri Service Research Laboratory
Fort Sam Houston, TX
http://www.wpafb.af.mil/afrl/
JPC Diagnoses:
1. Kidney: Pyelonephritis, chronic-active, suppurative, severe, multifocal to coalescing, with cellular and protein casts and moderate hydronephrosis.
2. Kidney: Interstitial nephritis, lymphoplasmacytic, mild, with tubular atrophy.
JPC Comment:
The contributor provides an excellent overview of Proteus mirabilis, a bacterium blessed with many gifts, the most spectacular of which may be its remarkable and pleasingly alliterative swimmer-swarmer motility. This unique feature inspired the name of the Proteus genus upon its discovery in 1885 by the German pathologist Gustav Hauser.7
Hauser, a Greek mythology enthusiast, named the genus based on Proteus, the prophetic sea god, who appears in, among other writings, Homer’s Odyssey. In addition to herding seals for Poseidon – a fine enough avocation in itself – Proteus was omniscient, knowing all things past, present, and future. Proteus was loathe, however, to share his knowledge with others, and those who sought his counsel could only bide their time and attempt to capture Proteus during his midday nap and demand prophetic answers. Proteus, however, was not just your run-of-the-mill omniscient seal-herding deity; he could also change shape into lions, serpents, or even water in order to avoid capture.
The name Proteus also appeared in the 15th century Shakespeare play, The Two Gentlemen of Verona, where one of the main characters is so named because of his capricious, frequently-changing affections. The modern word “protean” also shares this derivation, and is defined by Merriam-Webster to mean “of or resembling Proteus in having a varied nature or ability to assume different forms; displaying great diversity or variety; versatile.”9 It was this culture context that led Hauser, who recognized the ability of Proteus mirabilis to differentiate from a short vegetative swimmer cell to an elongated, highly flagellated swarmer cell, to name the bacterium after Proteus, mythology’s famous shape-shifter.8
What Hauser couldn’t have know at the time, however, is that P. mirabilis can perform even more feats of molecular chicanery that allow it to evade the host immune system. P. mirabilis produces an IgA-degrading protease, named ZapA metalloprotease, that is able to completely degrade IgA.1 The bacterium produces ZapA during its transition from swimmer to swarmer, enabling it to neutralize the host’s mucosal immunity during a critical time in pathogenesis. One of the key characteristics of the swarmer cell is the exuberant expression of flagella of many different types. These flagella are encoded by three different flagellin genes which, via homologous recombination, allow individual bacteria to produce antigenically novel flagella capable of evading host immune defenses.1 Finally, the production of urease leads to the production of struvite and carbonate hydroxyapatite stones. Stone formation is typical of P. mirabilis infection and allows the bacterium to sequester itself and replicate within the stones, protected from the antibodies and antibiotics that mean it harm.1Conference participants found this slide a feast for the eyes and, as in all cases this week, full of description-rich details. Dr. Alves advised conference participants to structure slide descriptions by starting with the most severe lesion; if severity doesn’t provide an obvious starting point, one might structure the description around the presumed pathogenesis. In this case, the most severe pathology was in cortical tubules, which were characterized by large areas of both coagulative and lytic necrosis. Dr. Alves noted that many seemingly destroyed tubules retained their basement mebranes in a remarkable display of renal resilience. He also noted that many tubular lumina contain blue-gray, finely granular material that appear to be bacteria, but that did not show up on gram stain. Gram staining was repeated post-conference and the intratubular material again resisted gram staining. Despite the presence or absence of organisms on gram stain, this section provides an excellent example of a “gram-negative kidney,” with lesions similar to what would be expected with E. coli, Proteus mirabilis, Klebsiella, and other less-common gram negative organisms.
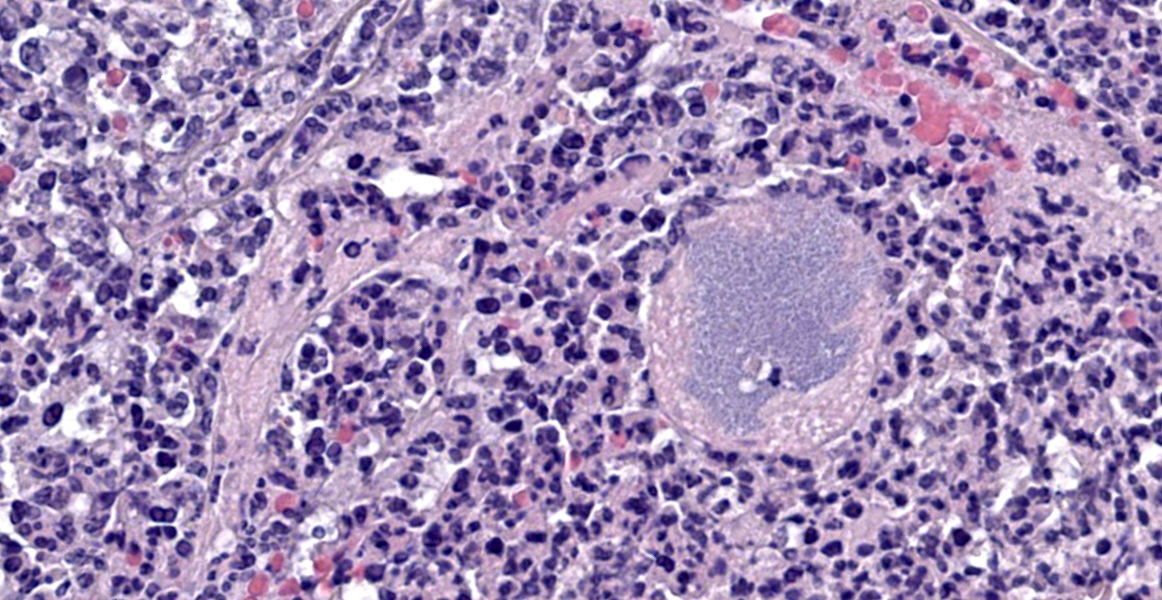
Dr. Alves also noted eosinophilic round inclusions within many renal tubular epithelial cells, interpreted as hyaline droplets. These well-documented droplets are produced by one of three distinct processes: 1) by sequestration of alpha2U-globulin in the lysosomes of male rats, 2) by histiocytic sarcoma, which produces excessive lysozyme that spills into the renal tubules and is resorbed, or 3) by overproduction of high molecular weight proteins. These three etiologies can only be differentiated with special stains.
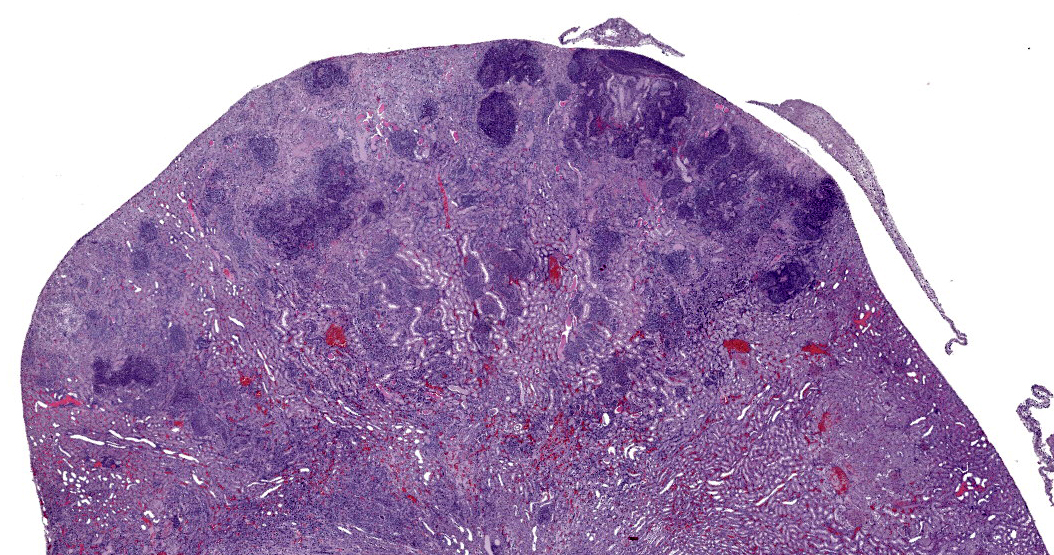
Finally, Dr. Alves noted an area near the renal pelvis that, in contrast to the vast majority of the kidney which is awash in neutrophils, contains an interstitial inflammatory infiltrate composed largely of lymphocytes and plasma cells. These chronic inflammatory cells separate and surround small renal tubules with attenuated epithelium, interpreted as tubular atrophy. Dr. Alves believes this area represents a second pathologic process distinct from the obvious bacterial infection. Though the age of the animal is not provided, chronic progressive nephropathy is a common lesion in older rats and could account for this focus of chronic inflammation. Conference participants felt this was a significant lesion and warranted capture in a second morphologic diagnosis.
References:
1. Coker C, Poore CA, Li X, Mobley HLT. Pathogenesis of Proteus mirabilis urinary ratct infection. Microbes Infect. 2000; 2(12):1497-1505.
2. Jones B, Young R, Mahenthiralingam E, Stickler D. Ultrastructure of Proteus mirabilis swarmer cell rafts and role of swarming in catheter-associated urinary tract infection. Infect Immun. 2004; 72 (7):3491-3950.
3. Marques C, Belas A, Franco A, Aboim C, Gama LT, Pomba C. Increase in antimicrobial resistance and emergence of major international high-risk clonal lineages in dogs and cats with urinary tract infection: 16 year retrospective study. J Antimicrob Chemother. 2018;73(2):377-384.
4. Mobley HLT. Virulence of Proteus mirabilis. In: Mobley H, Warren J, eds. Urinary Tract Infections: Molecular Pathogenesis and Clinical Management. ASM Press;1996:245-269.
5. Percy DH, Barthold SW, eds. Pathology of Laboratory Rodents and Rabbits. 4th ed. Blackwell Publishing; 2016:66.
6. Protean. 2023. In:Merriam-Webster.com. Retrieved December 9, 2023, from https://www.merriam-webster.com/dictionary.
7. Sellaturay SV, Nair R, Dickinson IK, Sriprasad S. Proteus: mythology to modern times. Indian J Urol. 2012;28(4):388-391.
8. Smith ML, Mellor D. Proteus mirabilis meningitis and cerebral abscess in the newborn period. Arch Dis Child. 1980; 55(4):308-310.
9. Zhang H, Diao H, Jia L, Yuan Y, Thamm D et al. Proteus mirabilis inhibits cancer growth and pulmonary metastasis in a mouse breast cancer model. PLoS One. 2017;12(12):e0188960.