Signalment:
Gross Description:
Histopathologic Description:
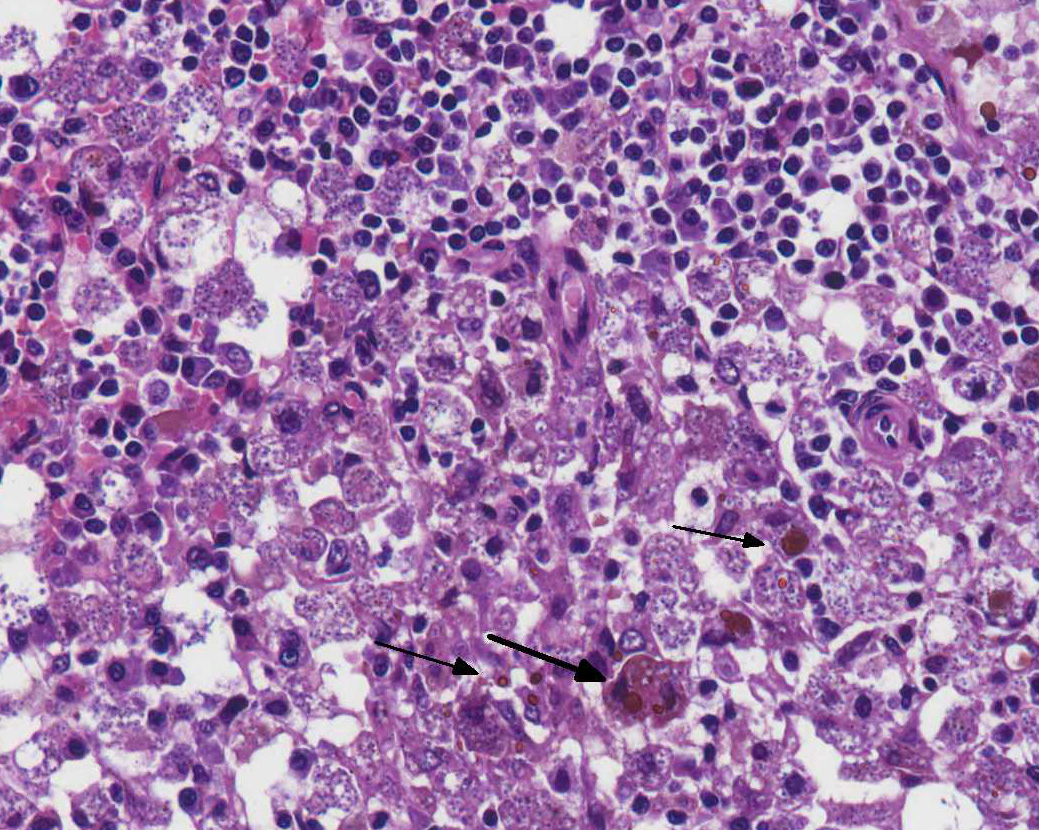
Bone marrow (proximal femur): The marrow parenchyma is completely replaced (myelopthisis) by numerous macrophages, moderate plasma cells and fewer lymphocytes. Macrophages contain myriad of basophilic structures similar to those described in the spleen (Fig. 4). Multifocally, are areas of karyorrhectic and cellular debris (lytic necrosis) containing occasional viable and degenerate neutrophils admixed with variable amounts of an eosinophilic beaded to fibrillar material (fibrin). There is multifocal hemosiderosis.
Tissues not submitted:
Lymph nodes present changes similar to those described in the spleen. Additionally, moderate lymphoid hyperplasia and increased plasma cell differentiation are observed. Macrophages containing myriad of amastigotes of Leishmania spp. are seen within lymphatic sinus, medullary cords, cortical and paracortical regions. In the liver there are multiple areas with loss of hepatocytes and infiltration of macrophages, plasma cells and lymphocytes. Some macrophages are loaded with amastigotes of Leishmania spp. In both organs there is moderate multifocal hemosiderosis.
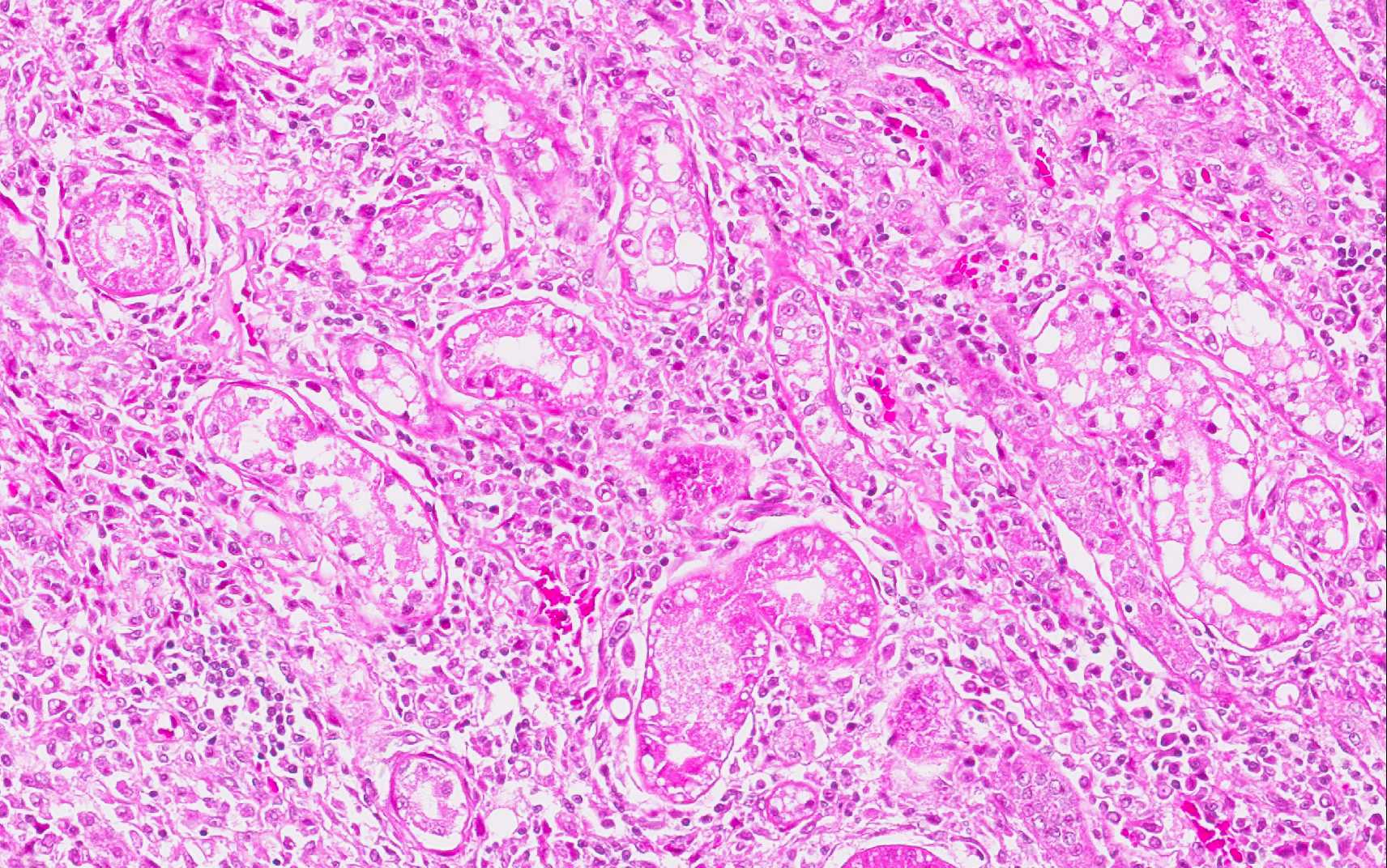
The kidneys presented with moderate membranous glomerulonephropathy. The Lumina of several tubules are ectatic, and contain aggregates of eosinophilic material (protein casts).
Amastigotes of Leishmania spp. were detected by immunohistochemistry (Streptavidin-biotin-peroxidase) in the spleen (Fig. 5) and in the bone marrow (Fig. 6 and 7).
Morphologic Diagnosis:
Spleen: Splenitis and perisplenitis, histiocytic and plasmacytic, diffuse, severe, with myriad of intrahistiocytic amastigotes, etiology consistent with Leishmania spp.
Bone marrow: Myelitis, histiocytic and lymphoplasmacytic, diffuse, severe, with myriad of intrahistiocytic amastigotes, etiology consistent with Leishmania spp.
Lab Results:
The dog was observed for two months and total blood cell count analyses were performed weekly. Overall, the dog had marked anemia, leukopenia, and thrombocytopenia. Clinical pathology results are presented in Table 1.
After the last transfusion, i.e. 62 days after the first hematological evaluation, the RBC, HCT, and hemoglobin values were within the reference range. Concomitantly with the fourth hem-atological evaluation, a bone marrow aspiration was performed. It was noticed the paucity of hematopoietic precursors, mainly from the erythroid lineage. Serological tests at this time resulted in positive ELISA and negative RIFI.
All hematological analyses revealed reduced numbers of platelets and lymphocytes. The numbers of monocytes remained below the reference range in most of the hematological exams.

Condition:
Contributor Comment:
The life cycle of Leishmania spp. involves two hosts a phlebotomine sand fly vector (genus Lutzomyia in the New World) and a mammal (including rodents, canids, or humans). Leishmania spp. occurs as flagellated, extracellular promastigotes in the gut of sand fly vectors. Infection occurs when a feeding sand fly deposits metacyclic promastigotes into the dermis of the host. In mammalian hosts, Leishmania spp. occur as amastigotes (2.0 to 3.0 μm in diameter) within mononuclear phagocytes in the skin, bone marrow, and visceral organs.(11) Although vector-borne is the most important route of transmission, CanL can also be transmitted in the absence of the invertebrate vector.(13)
CanL is manifested by a broad spectrum of clinical signs and degrees of severity. In the typical CanL case, history and physical examination include skin lesions, local or generalized lymphadenomegaly, emaciation, cachexia, splenomegaly, anorexia or increased appetite, lethargy, temporal muscle atrophy, exercise intolerance, polyuria/polydipsia, ocular lesions, epistaxis, onychogryphosis, lameness, vomiting, and diarrhea.(4) Serum biochemistry findings in dogs with clinical leishmaniosis include, most commonly, serum hypoproteinemia with hyperglobulinemia and hypoalbuminemia, resulting in a decreased albumin/globulin ratio. Mild increases of liver enzyme activities are frequent; however, grossly elevated liver enzyme activities, severe azotemia, or both, are found in only a minority of dogs with leishmaniosis. Proteinuria and some renal abnormalities develop in most dogs with this disease.(1)
Hematological parameters and the serum biochemical profile in L. infantum-infected dogs have limited diagnostic value. However, they can be useful biomarkers for evaluating the clinical progress of infected animals and may also contribute to the understanding of CanL pathogenesis. One the most remarkable characteristics of CanL-associated hematological disorders is anemia, characterized as non-regenerative normocytic or normochromic. The leucocyte alteration in the blood of symptomatic dogs includes leucopenia characterized by monocytopenia, lymphopenia, and eosinopenia.(1) The pathogenesis of hematological changes in both red and white blood cells is often related to bone marrow disorders associated with diminished erythropoiesis due to intense bone marrow parasitism. In addition, anemia can be related to reduced plasma iron due to abnormal iron retention by macrophages and increased levels of hepcidin, typical of anemia of chronic diseases. Anemia could also be related to increased hemolysis in enlarged spleen and liver associated with inflammatory response to L. infantum and to decreased production of erythropoietin by damaged kidneys.(7)
Severely affected dogs are usually cachectic and suffer from muscle atrophy. The skin and hemolymphatic organs are primarily affected. Generalized lymphadenomegaly and splenomegaly are usually present. Hepatomegaly may be present, but is less common. Small nodular foci of inflammation may develop in various organs, including the skin and the kidneys. Mucosal ulcerations in the nasal cavity, stomach, intestine, and colon are occasionally observed. The typical histopathologic finding in the majority of affected tissues is an inflammatory reaction associated with macrophages in the presence or absence of Leishmania amastigotes. Amastigote numbers may vary from very few organisms within macrophages to large numbers in rarer events. Lymphoplasmacytic inflammation is also common in dogs with leishmaniasis.(1) Additionally, renal disease due to glomerulonephritis and interstitial nephritis is commonly associated with CanL due to Leishmania infantum.
Diagnosis of canine leishmaniasis is based on the presence of clinical signs together with positive specific antibody assay. Infection can be confirmed by demonstration of the parasites (amastigotes) on touch prep stained (Wright-Giemsa) slides or in cultures of tissue aspirates or biopsy specimens of the spleen, liver, bone marrow, or lymph nodes.(1) Immunohistochemistry is a useful tool that significantly increases the sensitivity of histopathology for detecting amastigotes, and it is a genus-specific assay to confirm the diagnosis.(12)
JPC Diagnosis:
1. Spleen, red pulp: Splenitis, histiocytic and plasmacytic, diffuse, marked with numerous intracellular amastigotes.
2. Spleen: Reticuloendothelial hyperplasia with erythrophagocytosis and hemo-siderosis.
3. Spleen: Extramedullary hematopoiesis.
4. Bone marrow: Myelitis, histiocytic and plasmacytic, diffuse, marked with numerous intracellular amastigotes.
Conference Comment:
The conference histologic description was aligned very closely with the contributors description above. Conference participants were struck by the marked degree of effacement of both the splenic red pulp and bone marrow.
We thank the contributor for providing clinical pathology data with the submission, which greatly enhances the teaching value of the case. Participants discussed the third hematological evaluation and whether the anemia is regenerative or nonregenerative, but without a reticulocyte count it is difficult to determine with certainty. The presence of nucleated red blood cells (metarubricytosis) can be indicative of regeneration with concurrent reticulocytosis, but nucleated red blood cells can also be present in other conditions such as bone marrow damage from inflammation or necrosis, lead poisoning and due to splenectomy among other things. A normal MCV would support a nonregenerative anemia; additionally, the pancytopenia and histologic changes in the bone marrow also provide support for a nonregenerative anemia. Elevated ALT and AST are indicative of mild hepatocellular damage, which correlates with the histopathologic description mentioned above by the contributor in the tissues not submitted. The total protein is normal but a decrease in albumin and increase in globulin is likely present in this case, based on the degree of infection and ongoing inflammation, which is a common pattern in dogs afflicted with leishmaniasis, as mentioned above by the contributor.
References:
1. Baneth G, Solano-Gallego L. Leishmaniasis. In: Greene CE, ed. Infectious diseases of the dog and cat. 4th ed. Philadelphia, US: Elsevier Saunders; 2012:735-748.
2. Cooper BJ, Valentine BA. Muscle and Tendon. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol 1. St. Louis, MO: Elsevier; 2015:240.
3. Diniz SA, Silva FL, Carvalho Neta AV, et al. Animal reservoirs for visceral leishmaniasis in densely populated urban areas. J Infect Dev Ctries. 2008; 2:24-33.
4. Koutinas AF, Koutinas CK. Pathologic mechanism underlying the clinical findings in canine Leishmaniosis due to Leishmania infantum/chagasi. Vet Pathol. 2014;51(2)527-538.
5. Mauldin EA, Peters-Kennedy J. Integumentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol 1. St. Louis, MO: Elsevier; 2015:663.
6. Minist+â-¬rio da Sa+â°de. Manual de vigil+â-óncia e controle da leishmaniose visceral. Bras+â-¡lia, BR: Editora MS; 2006.
7. Nicolato RC, Abreu RT, Roatt BM, et al. Clinical forms of canine visceral Leishamniasis in naturally Leishmania infantum-infected dogs and related myelogram and hemogram changes. Plos One. 2013;8(12):1-9.
8. Papa DN. Perfil epidemiologico da Leishmaniose Visceral em c+â-úes diagnosticasdos no Laborat³rio da Escola de Veterin+â-íria da Universidade Federal de Minas Gerais Belo Horizonte 2004 a 2008. Masters degree dissertation, Escola de Veterin+â-íria, Universidade Federal de Minas Gerais, 2010.
9. Ready PD. Epidemiology of visceral leishmaniasis. Clin Epidemiol. 2014; 6:147-154.
10. Romero GAS, Boelaert M. Control of visceral Leishmaniasis in Latin America A systematic review. Plos Negl Trop Dis. 2010;4(1):1-17.
11. Solano-Gallego L, Mir³ G, Koutinas A, et al. LeishVet guidelines for practical management of canine leishmaniosis. Parasite Vect. 2011;4:86.
12. Tafuri WL, Santos RL, Arantes MRE, et al. An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. J Immunol Methods. 2004; 292:17-23.
13. Turchetti AP, Souza TD, Paix+â-úo TA, Santos RL. Sexual and vertical transmission of visceral leishmaniasis. J Infect Dev Ctries. 2014; 8:403-407.
14. Valli VEO, Kiupel M, Bienzle D. Hematopoietic System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol 3. St. Louis, MO: Elsevier; 2015:1