Signalment:
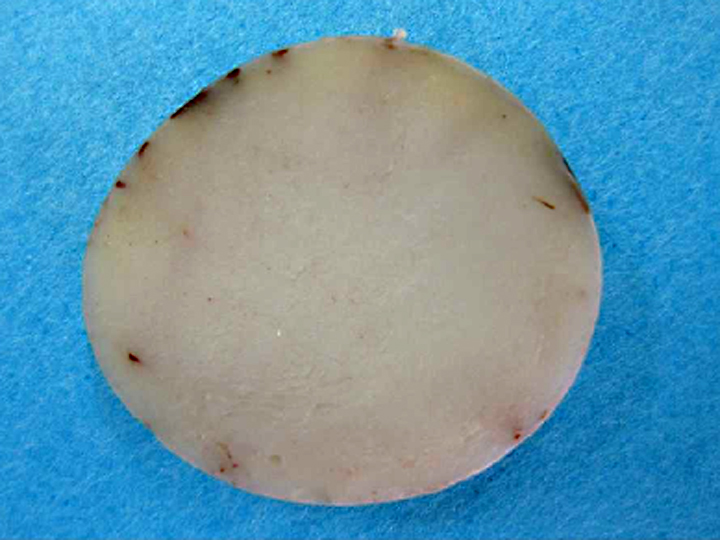
Gross Description:
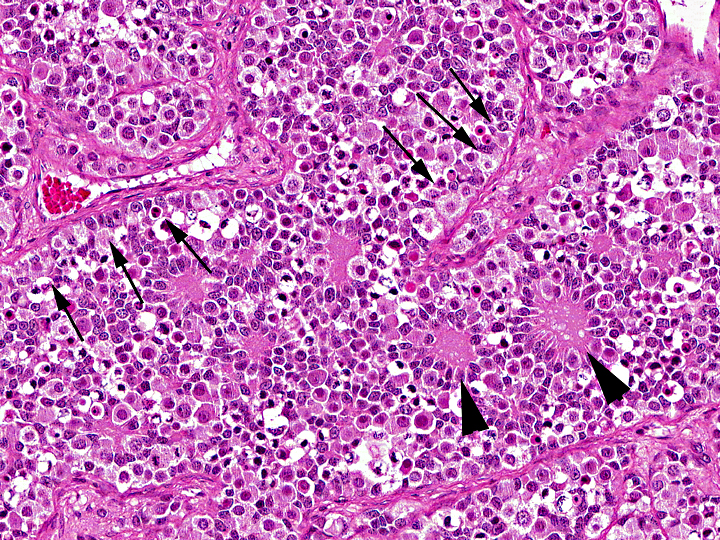
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
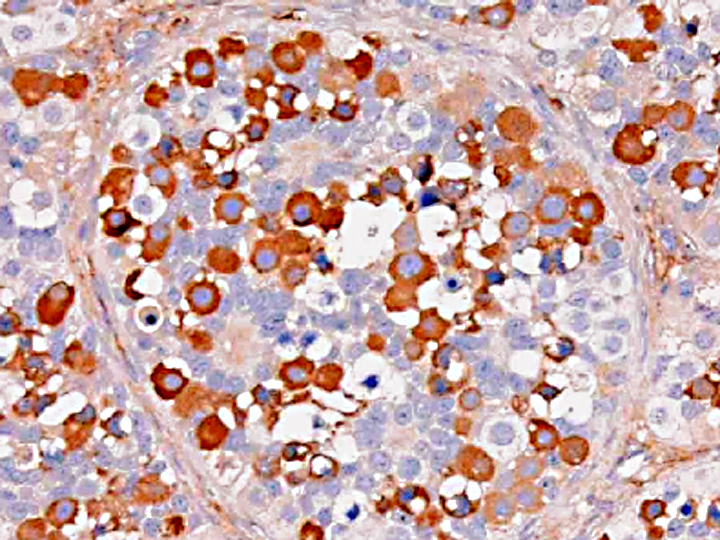
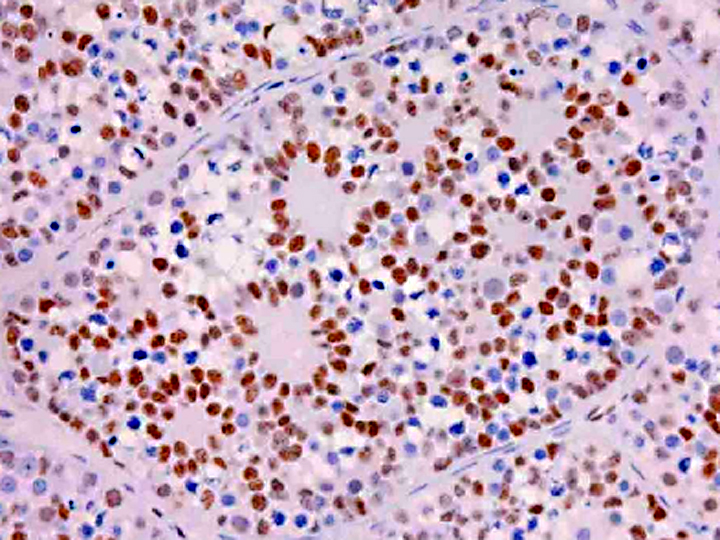
Gonadoblastoma is histomorphologically defined as a tumor composed of two principal cell types: germ cell components similar to those of seminoma and sex cord components similar to Sertoli cells. In this case, immunohistochemical examination revealed both the germ cell derivative of the large round cells (c-kit and PLAP positivity) and the sex cord nature of the spindle cells (vimentin and WT-1 positivity) in addition to characteristic morphological features. Furthermore, the ultrastructural pattern of eosinophilic amorphous bodies that are comprised of whorled laminae is already verified in canines(6) and those of human gonadoblastomas.
The differential diagnosis includes mixed germ cell-sex cord-stromal tumor (MGSST) and sex cord tumor with annular tubules (STAT). MGSST generally lacks the discrete tubular structures seen in gonadoblastoma, and usually shows proliferative activity in the sex cord component, , because unlike in gonadoblastoma, germ cells in MGSST are thought to be non-neoplastic and entrapped by neoplastic sex cord-stromal tumor. The histomorphological features showing characteristic three typical patterns (i.e.coronal, follicular, and Call-Exner-like) of sex cord elements within the discrete tubules is most characteristic and diagnostic for gonadoblastoma. In a gonadoblastoma, more than one pattern is usually found in an individual tubule(6). The presence of these patterns supports the diagnosis of gonadoblastoma in this case. STAT has a growth pattern similar to gonadoblastoma and contains eosinophilic amorphous bodies and frequently calcified material, but lacks a germ cell component. Our case is conclusively distinguished from these diagnoses.
Gonadoblastoma typically occurs within dysgenic gonads in children or young adults. Approximately 80% of cases occur in phenotypic females, and most of the remaining 20% are in phenotypic male pseudohermaphrodites(6). In more than 90% of patients with gonadoblastoma, a Y chromosome or fragment can be identified(6). However, it has been found in the testes of normal men(2). Our rabbit has no clinical symptoms such as alopecia, feminization, or cryptorchidism. Our case was apparently sexually intact judging from the intact right testis and normal development of genital organs, and has no dysgenic gonads. However, it remains unclear whether this rabbit had Y chromosome fragments or not, because karyotypic analysis was not done.
JPC Diagnosis:
Conference Comment:
Placental alkaline phosphatase (PLAP) only stains neoplastic germ cells, in addition to rare somatic epithelial malignancies, and not normal germ cells, which is useful in distinguishing this neoplasm from a mixed germ cell-sex cord-stromal tumor(11). The transcription factor Wilms tumor-1 (WT-1) protein, in addition to staining nephroblastomas, is a marker of M+â-+llerian epithelial origin, and regulates the k-ras oncogene, which plays an important role in tumor suppression. Loss of WT-1 drives cells expressing oncogenic k-ras toward a senescence program, and inhibits the progression of certain types of neoplasia(10). Sex cord stromal tumors are among several neoplasms in veterinary medicine that co-express vimentin and cytokeratin (AE1/AE3); others include canine prostatic carcinoma, feline bronchogenic adenocarcinoma, synovial cell sarcoma, ciliary body adenoma, renal carcinoma, amelanotic melanoma, meningioma, mesothelioma, and anaplastic carcinoma. However, due to the growing list of neoplasms that can express both cytokeratin and vimentin, the diagnostic utility of vimentin is increasingly coming into question(1).
References:
2. Chapman WH, Plymyer MR, Dresner ML: Gonadoblastoma in an anatomically normal man: a case report and literature review. J Urol 144:1472-1474, 1990
3. Maratea KA, Ramos-Vara JA, Corriveau LA, Miller MA: Testicular interstitial cell tumor and gynecomastia in a rabbit. Vet Pathol 44:513-517, 2007
4. Reis-Filho JS, Ricardo S, G+â-ñrtner F, Schmitt FC: Bilateral gonadoblastomas in a dog with mixed gonadal dysgenesis. J Comp Pathol 130:229-233, 2004
5. Scully RE: Gonadoblastoma. A review of 74 cases. Cancer 25:1340-56, 1970
6. Talerman A, Roth LM: Recent advances in the pathology and classification of gonadal neoplasms composed of germ cells and sex cord derivatives. Int J Gynecol Pathol 26:313-321, 2007
7. Turk JR, Turk MA, Gallina AM: A canine testicular tumor resembling gonadoblastoma. Vet Pathol 18:201-7, 1981
8. Hou-Jensen K, Kempson RL. The ultrastructure of gonadoblastoma and dysgerminoma. Human Pathol. 1974;5(1):79-91.
9. Suzuki M, et al. Testicular gonadoblastoma in two pet domestic rabbits (Oryctolagus cuniculus domesticus). J Vet Diag Invest. 2011;23(5):1028-32.
10. Vincent S, et al. Wilms tumor 1 (WT1) regulate KRAS-driven oncogenesis and senescence in mouse and human models. J Clin Invest. 2010;120(11):3940-52.
11. Wick MR. Immunohistology of melanocytic neoplasms. In: Dabbs DJ. Diagnostic Immunohistochemistry, Theranostic and Genomic Applications. 3rd ed. Philadelphia, PA:Saunders Elsevier; 2010:191.