Wednesday Slide Conference, Conference 3, Case 4
Signalment:
18 year-old, male, rhesus macaque, Macaca mulatta
History:
During a physical examination, a mass was found at the back of the mouth and was biopsied. A week later, based on the biopsy result, the monkey was euthanized.
Gross Pathology:
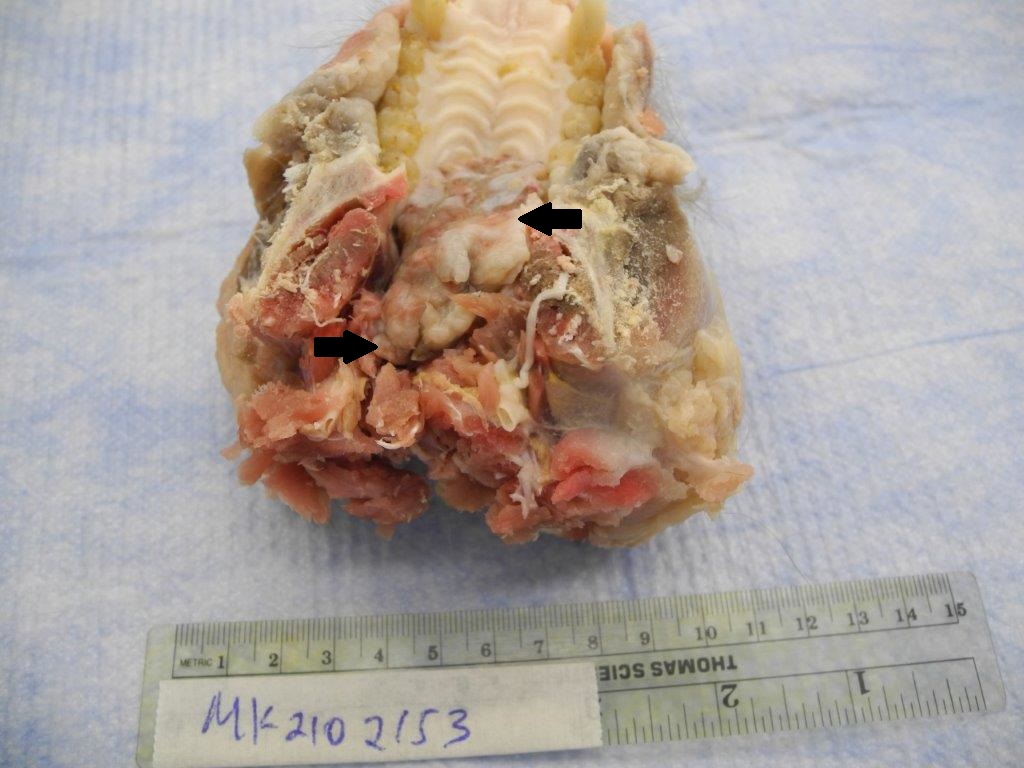
A 4 x 2 cm mass extended from the caudal aspect of the hard palate into the soft palate and pharynx. The mass was soft with an irregular surface that was mottled light and dark gray. The draining lymph nodes appeared to be enlarged. Photo of fixed tissue: arrows delineate the extension of the mass from the ulcerated hard palate into the pharynx.
The liver was markedly enlarged and diffusely pink and had a white texture on cut section. Along the apical margins of the liver were white well demarcated firm foci that also were waxy in texture. Lungs, heart, spleen, kidneys and GI were grossly normal.
Microscopic Description:
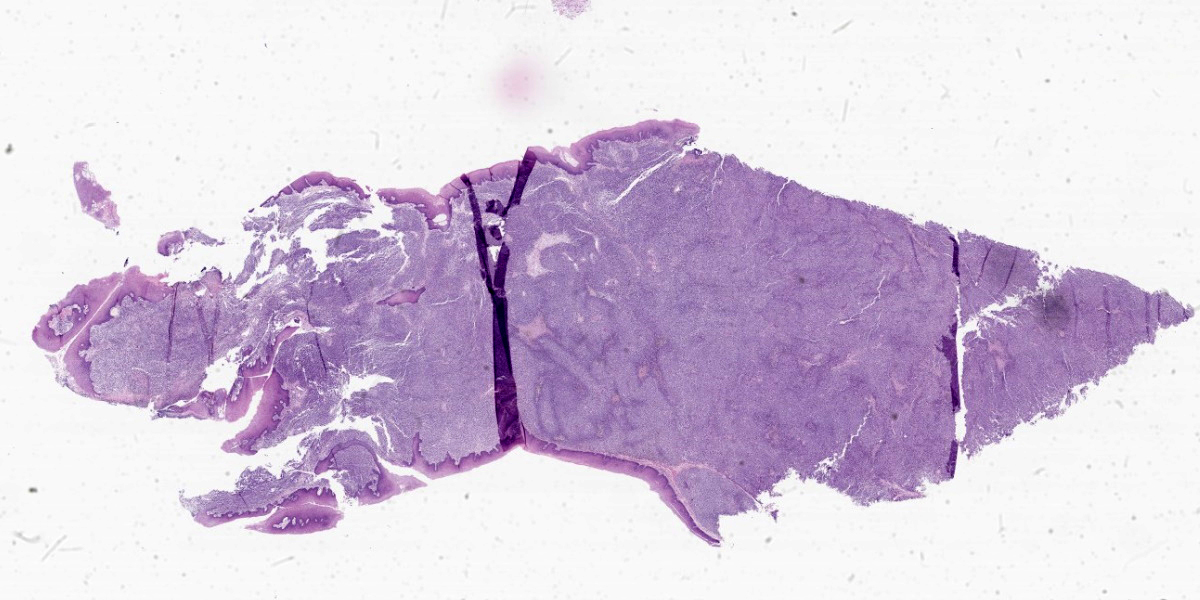
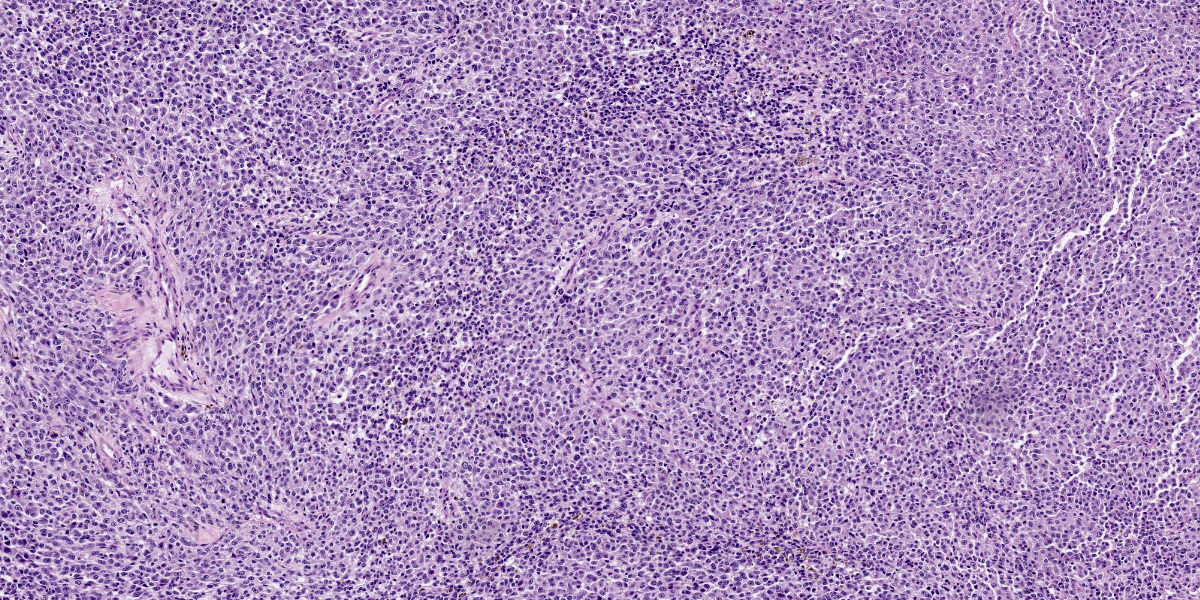
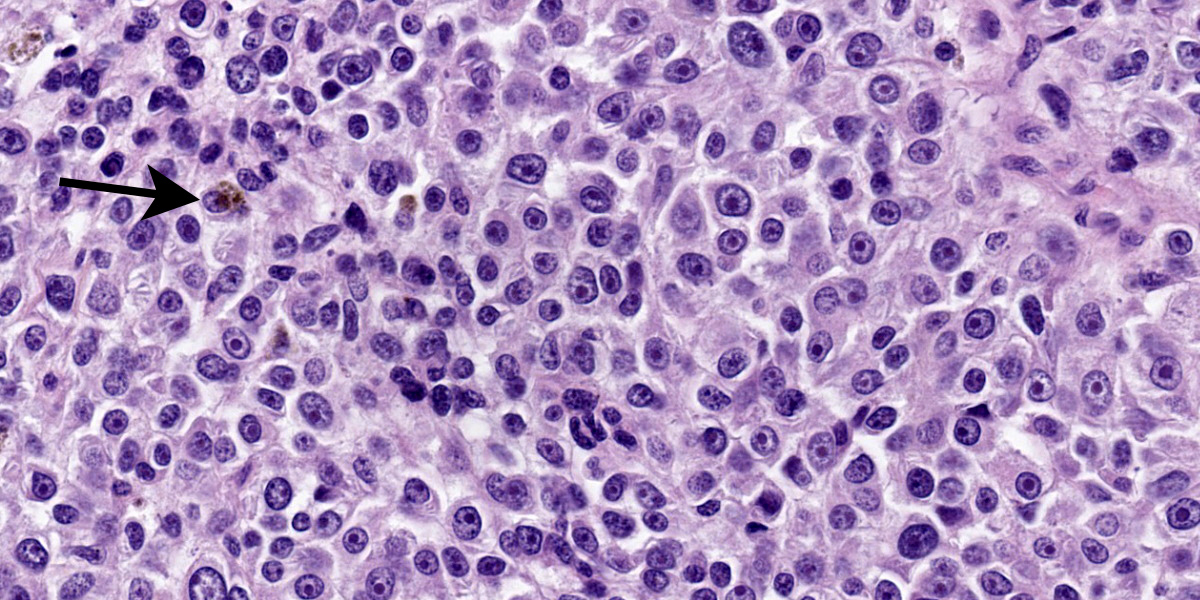
Moderately pleomorphic neoplastic cells expanded the submucosa and extended to the cut edges of the sections. The cells were round to oval to stellate and ranged in size from 15-50um. Nuclei were round to oval and had prominent nucleoli. Cells had abundant wispy cytoplasm with small vesicles and a few had faint brown pigment granules. Mitoses averaged 2/HPF although some fields had as many as 5 mitotic figures. Some of the mitoses had an unusual appearance. In addition, hyphae consistent with candida and bacterial cocci were present in ulcerated areas [not present in all slides]
Submandibular lymph nodes were largely effaced by similar cells. A section of lung had microscopic metastasis.
Liver: The liver had severe amyloidosis with tumorous deposits at the apical margins. Heart, spleen, kidney and salivary glands were normal.
Special stains:
Fontana-Masson: biopsy, tumor, and lymph node had scattered positive cells.
Giemsa: (-)
Immunohistochemistry:
S-100, SOX-10, Melan A, PNL, HM45: (+)
Cytokeratin AE1/AE3: (-)
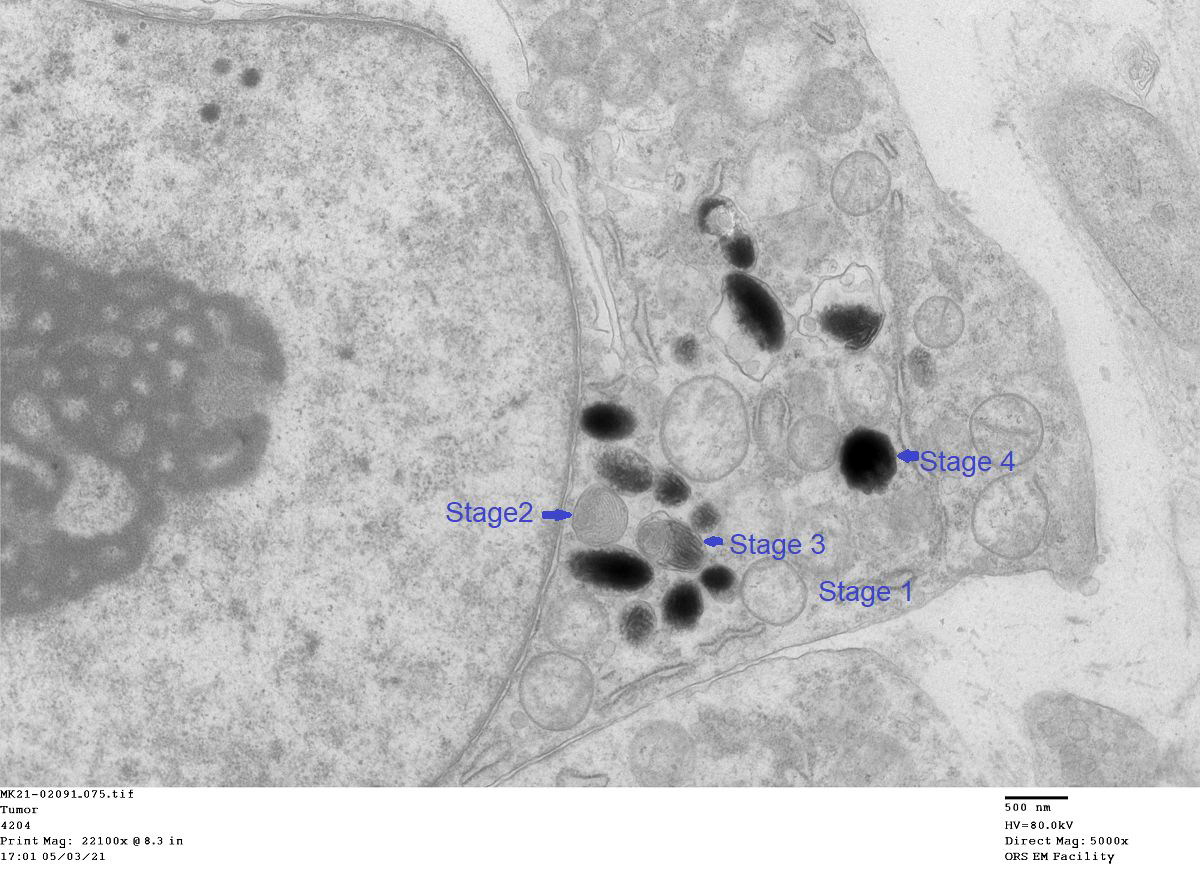
TEM: One percent of the tumor cells were positive for melanin and `contained 1 to 50 granules. All four stages of melanin granules were present with stages 3 and 4 predominating. The enclosed image shows all four stages.
Contributor’s Morphologic Diagnosis:
Amelanotic melanoma
Contributor’s Comment:
Melanocytes arise from neural crest cells and reside not only in the basal cells of the epidermis and hair follicles, but also the eyes and meninges. Melanin synthesis occurs in melanosomes, lysosome-like organelles in melanocytes. Two major melanin pigments synthesized are pheomelanin (red/yellow in color) and eumelanin (brown/black). Both pigments arise from L-tyrosine which, when acted upon by tyrosinase, leads to DOPA (dihydroxyphenylalanine) formation. Production of DOPA from L-tyrosine is the rate limiting step in melanogenesis.3 Oxidation and polymerization of DOPA leads to the formation of pheomelanin. Further enzymatic action by tyrosinases produces eumelanin. The percentage of eumelanin and pheomelanin determines the color of the skin/hair.3,5
Packaging of melanin into melanosomes occurs in four identifiable stages as seen by electron microscopy. Early melanosome stages I and II do not contain melanin but fibers formed during these stages give melanosomes their ovoid shape. Deposition of melanin begins in stage III and completely fills the melanosome in stage IV melanosomes.5 Once formed, melanosomes are transferred to keratinocytes and are moved into the supranuclear area to form melanin caps which protect nuclei against UV damage. Melanin degrades as keratinocytes undergo squamous maturation.3,5
Melanoma is called the “great imitator” as the cells can have epithelioid, spindloid, clear, signet ring–like, myxoid, desmoplastic, rhabdoid, ballooning, and plasmacytoid forms.7,9,10 Additional features of melanoma include high mitotic rate, unusual morphology of mitotic figures and the presence of junctional change by neoplastic cells.6,12
Some common stains used for diagnosis are Fontana-Masson which stains melanin granules brown in tumors that have little pigmentation. Bleach clears melanin in deeply pigmented tumors to examine the morphology of the cells. Giemsa is another stain to rule out mast cell tumors
Since melanomas, and other pigmented masses, may express more than one marker and immunohistochemistry can stain more than one cell type, including non-neoplastic cells, “cocktails” of markers are used to make diagnoses, identify micrometastases in sentinel
lymph nodes, determine prognosis, and develop treatment strategies.2,3,7 Specific markers include:
Cytokeratin: expressed in epithelial origin tumors.
S-100: is expressed in all subtypes of melanoma and identifies cells derived from the neural crest.7 S-100 may also be expressed in other tumors.
Melan-A: (aka melanoma-associated antigen recognized by T cells) plays an important role in the formation of stage II melanosomes which have structural proteins.2,7
MITF: (microphthalmia-associated transcription factor) is the central regulator of melanogenesis. It is essential for melanocyte development and regulates genes for melanogenesis, cell survival, and differentiation.3,7 MITF stain is sensitive but not specific. It is used as part of a cocktail because the staining pattern is nuclear and most other stains are cytoplasmic.3,7
HMB-45: (human melanoma black) is associated with the structural organization of melanosomes and with the fibrillar matrix and the maturation in stages I to II melanocytes.7
SOX10: (sry-related HMg-box gene10) is a nuclear transcription factor that regulates the differentiation of neural crest progenitor cells into melanoblasts and melanocytes.3,7
A pigmented, perioral melanoma with metastases to the lymph nodes, lung, liver, and kidney has been reported in a mountain gorilla.4 Oral melanoma has not been reported in macaques and reports of melanoma involving other sites are rare. There is one report of melanoma in the choroid with no metastases in a cynomolgus macaque1 and one report of cutaneous melanoma with metastases to local lymph nodes, also in a cynomolgus macaque.8
In humans, melanoma can be separated into three categories based on the amount of sun exposure: high cumulative sun damage, low cumulative sun damage and sun protected. Sun protected areas are further divided into acral (i.e. palms of the hands, soles of the feet, and the nails), mucosal/genital, uveal and CNS. The type and number of mutations can vary based on the location of the primary tumor.2,13
While the incidence of cutaneous melanoma has increased over the last 50 years, the incidence of mucosal melanoma has remained fairly constant.2,13 Mucosal membrane melanomas are rare and are more aggressive with a less favorable outcome compared to cutaneous melanomas. The low survival rate is due to lack of clinical signs and the likelihood that melanoma has already spread to local lymph nodes at the time of diagnosis.2,9,12 In addition to local lymph nodes, melanoma tends to metastasize to the lungs and liver.2,13
Malignant melanoma has been reported to develop in a variety of mammalian and nonmammalian species including dog, rabbit14, shark, parrot, pig and monkey.2,13 In the dog, malignant melanoma is the most common oral tumor9, is locally aggressive and tends to metastasize to local lymph nodes and lung.9,10 Breeds that are more likely to develop oral melanoma are Poodle, Golden and Labrador retrievers, Rottweilers and Yorkshire terriers.9
Some aspects of canine oral melanoma are similar to humans. In both, the etiology is unknown, clinical course is aggressive and definitive treatment for oral melanoma is lacking.2,9 The appearance of the neoplastic cells is variable and combinations of immunohistochemistry markers are used to diagnose the subtype of melanoma more definitively.7,9 For example, a cocktail of Melan-A, PNL2, TRP-1(tyrosinase related protein-1), and TRP-2 (tyrosinase related protein-2) has been found to be useful in the diagnosis of amelanotic melanoma in dogs.10
Treatment may include surgical excision, radiotherapy, and chemotherapy but none have been particularly effective as the behavior of oral melanoma differs from that of cutaneous melanoma.2,9 Immunotherapy with checkpoint inhibitors that allow T-cells to become activated against tumor cells has been successful in treating cutaneous melanoma in man, but less so for oral melanoma.2 In dogs, the potential of identifying checkpoint inhibitors and developing immunotherapy is being explored.9,11
Contributing Institution:
NIH
9000 Rockville Pike
Building 28A, Room 117
Bethesda, MD 20892
JPC Diagnosis:
Oral cavity: Melanoma.
Oral mucosa: Stomatitis, ulcerative, focally extensive, marked with numerous yeast, pseudohyphae, hyphae, and cocci.
JPC Comment:
We repeated the same list of IHC and special stains outlined by the contributor and got nearly identical results. In particular, neoplastic cells were strongly and diffusely immunoreactive for PNL2, Melan-A Red, and SOX10, though they did not react with HMB-45 which may reflect a difference in lab assay performance. With our Fontana-Masson stain applied, approximately 5-10% of neoplastic cells have intracytoplasmic black granules (melanin) which is also evident on H&E to a lesser extent (figure 4-4). Conference participants felt that these results were consistent with the diagnosis of a melanoma but felt that there were enough granules evident on H&E alone to forgo the ‘amelanotic’ label. The exact cutline for having little melanin present may vary per pathologist and many hypopigmented lesions probably fall into the amelanotic camp. At the JPC, we typically withhold the qualifier of ‘amelanotic’ for cases that have no discernable melanin on H&E. To our knowledge, published reports of oral melanoma in the non-human primate remain rare in line with what the contributor notes.
The inclusion of electron microscopy in this case workup is a welcome addition (figure 4-5). Recognizing melanosomes on EM is helpful as they characteristically have an oblong shape that Dr. Bruce Williams swears looks like a watermelon (or perhaps a football) in second of the four phases of melanin synthesis that contributor highlights.
In addition, conference participants honed in on a second aspect of this case that the contributor may have curtailed given the lengthy write up on melanoma they provided. In the bottom left (approximately 8 o’clock on figure 4-2), the oral mucosa is ulcerated, edematous, and has several small blood vessels with clear indications of vasculitis. Immediately adjacent, there are numerous fungal mats that contain yeast, pseudohyphae, and hyphae as well as colonies of cocci. Participants felt that these changes were consistent with ulcerative stomatitis secondary to Candida. In the history provided for this case, there was no mention of immunosuppression (i.e. SHIV infection) or antibiotic use though both of these are reasonable differentials for this lesion alone. After careful discussion, we added a second morphologic diagnosis for this case (a rarity for neoplasms) as we felt that this process was not directly connected to the melanoma in this case given the lack of junctional activity and condition of the remaining portion of the oral mucosa. Lastly, tissue ID for this case was aided by the presence of plant material (food) in section, though other similar tissues with stratified epithelium in a primate include the vagina and sex skin.
References:
- Albert DM, Dubielzig RR, Li Y, et al. Choroidal Melanoma Occurring in a Nonhuman Primate. Arch Ophthalmol. 2009;127(8):1080–1082.
- Dika E, Lambertini M, Pellegrini C, et al.. Cutaneous and Mucosal Melanomas of Uncommon Sites: Where Do We Stand Now? J Clin Med. 2021 Jan 28;10(3):478.
- D’Mello SA, Finlay GJ, Baguley BC, Askarian-Amiri ME. Signaling Pathways in Melanogenesis. Int J Mol Sci. 2016 Jul 15;17(7):1144.
- Kambale Syaluha E, Zimmerman D, Ramer J, et al. Metastatic perioral melanoma in a wild mountain gorilla (Gorilla beringei beringei). J Med Primatol. 2021 Jun;50(3):197-200.
- Lambert MW, Maddukuri S, Karanfilian KM, Elias ML, Lambert WC. The physiology of melanin deposition in health and disease. Clin Dermatol. 2019 Sep-Oct;37(5):402-417.
- Mauldin EA, Peters-Kennedy J. Integumentary System. In: Maxie MG, ed. Jubb, Kennedy & Palmer's Pathology of Domestic Animals. Vol 1. 6th ed. St. Louis, MO: Elsevier; 2016:720-722.
- Ordóñez, NG. Value of melanocytic-associated immunohistochemical markers in the diagnosis of malignant melanoma: a review and update. Hum Pathol. 2014 Feb;45(2):191-205.
- Pellegrini G, Bienvenu JG, Meehan JT, et al. Cutaneous melanoma with metastasis in a cynomolgus monkey (Macaca fascicularis). J Med Primatol. 2009 Dec;38(6):444-7.
- Prouteau A, André C. Canine Melanomas as Models for Human Melanomas: Clinical, Histological, and Genetic Comparison. Genes (Basel). 2019 Jun 30;10(7):501.
- Smedley RC, Lamoureux J, Sledge DG, Kiupel M. Immunohistochemical diagnosis of canine oral amelanotic melanocytic neoplasms. Vet Pathol. 2011 Jan;48(1):32-40.
- Stevenson VB, Perry SN, Todd M, Huckle WR, LeRoith T. PD-1, PD-L1, and PD-L2 Gene Expression and Tumor Infiltrating Lymphocytes in Canine Melanoma. Vet Pathol. 2021 Jul;58(4):692-698.
- Thomas NE, Kricker A, Waxweiler WT, et al. Comparison of Clinicopathologic Features and Survival of Histopathologically Amelanotic and Pigmented Melanomas: A Population-Based Study. JAMA Dermatol. 2014;150(12):1306–1314.
- Van der Weyden L, Brenn T, Patton EE, Wood GA, Adams DJ. Spontaneously occurring melanoma in animals and their relevance to human melanoma. J Pathol. 2020;252(1):4-21.
- Zerfas PM, Brinster LR, Starost MF, Burkholder TH, Raffeld M, Eckhaus MA. Amelanotic melanoma in a New Zealand White Rabbit (Oryctolagus cuniculus). Vet Pathol. 2010 Sep;47(5):977-81.