Signalment:
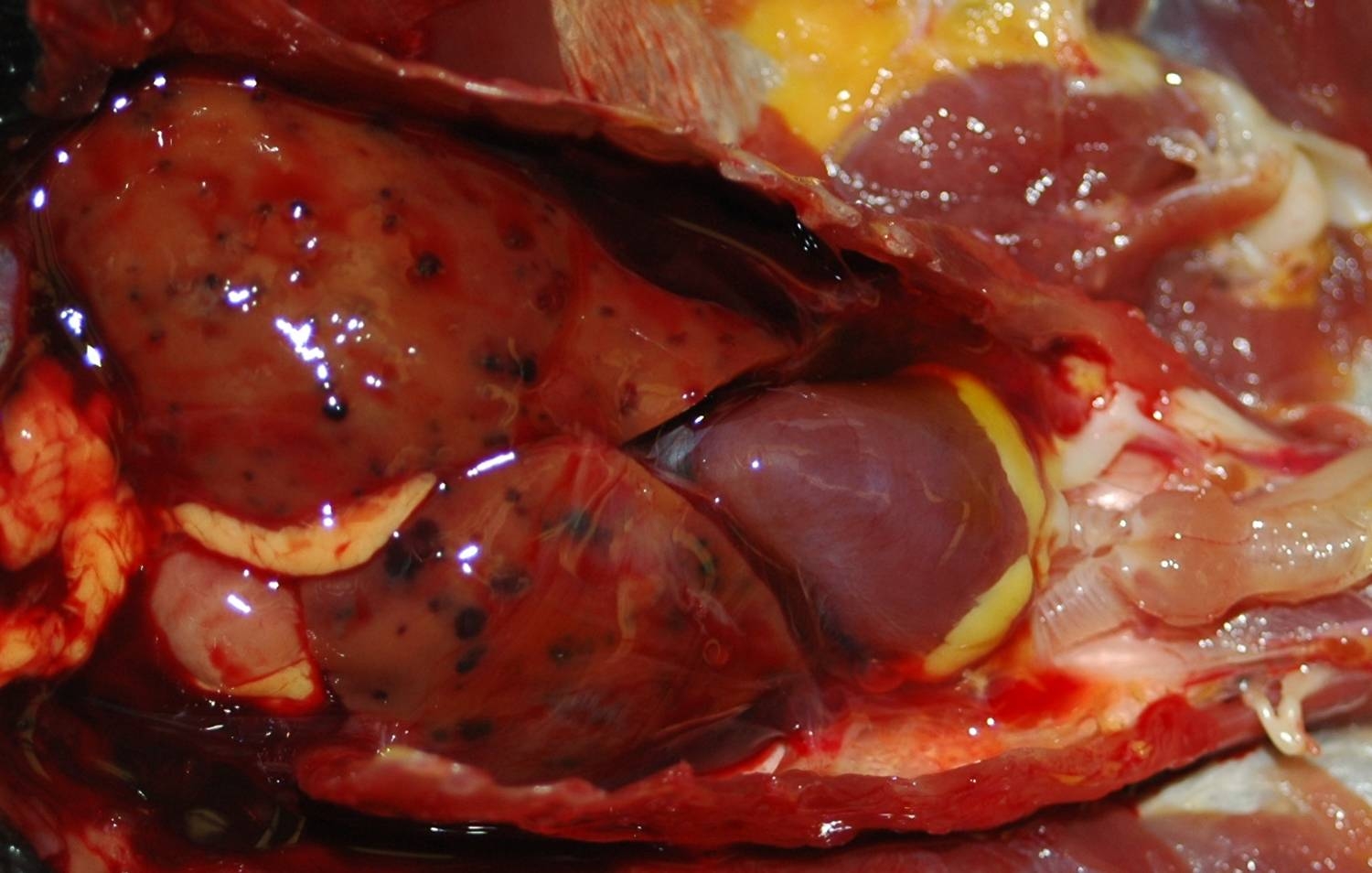
Gross Description:
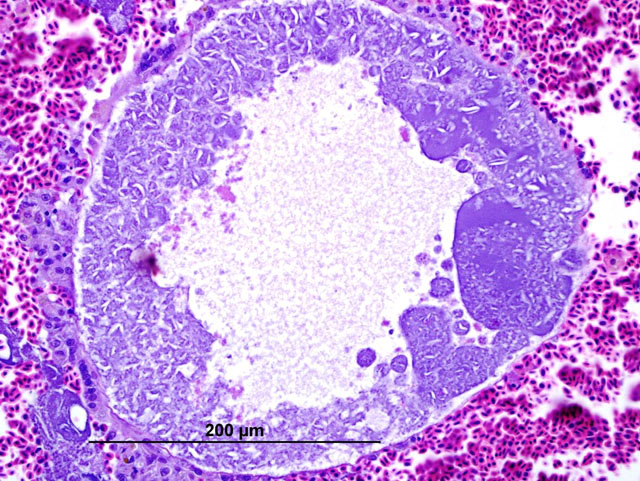
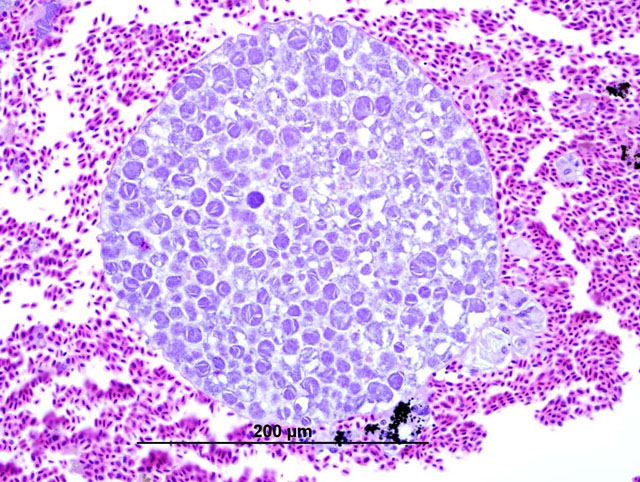
Histopathologic Description:
Morphologic Diagnosis:
Liver: Moderate diffuse hemosiderosis
Condition:
Contributor Comment:
Hemoparasitism in birds typically consists of 3 genera of Apicomplexan parasites in the family Plasmodiidae: Leukocytozoon, Haemoproteus and Plasmodium.4 These protozoa can cause severe clinical disease, or hosts may be asymptomatic. Haemoproteus and Plasmodium are distributed almost worldwide,16 with high species diversity. Over 120 species of Haemoproteus have been reported in birds.4 Haemoproteus spp. are characterized by schizogony (merogony) within visceral endothelial cells (typically the lung, liver or spleen), with gametocyte development in circulating erythrocytes.4,5 Biting flies, characteristically louse flies (Hippoboscidae) and biting midges (Ceratopogonidae) transmit the protozoa. In our cases, Ceratopogonidae are considered to be the more likely vector. Sexual development of Haemoproteus occurs in the intermediate host (insects), with asexual development in the bird.5 Clinical disease is typically associated with anemia due to erythrocytic parasitism, frequently in a compromised or immunocompromised host. Few species of Haemoproteus are reported to induce clinical disease, including H. meleagridis in turkeys, H. mettionis in ducks and geese, and H. columbae in pigeons and doves.13
In these cases, disease manifestation is presumed to occur with the pre-erythrocytic stages, rather than circulating intra-erythrocytic gametocytes, as no gametocytes were identified with lung impression smears (a representation of peripheral blood). Pre-erythrocytic schizont stages of Haemoproteus spp. have been reported to occur in many organs including the lung, liver, spleen, heart, kidney and cecum.2 Schizont development within capillary endothelial cells and myofibroblasts suggests that the parasite can use a variety of host cells.2 The reason for the apparent tropism to the liver in our cases is unknown. Because of the location of the schizont formation and sequelae to hepatic parenchymal disruption (hemorrhage and hemocoelom), it is plausible that there is not enough time for erythrocytic forms to be identified. Experimental Haemoproteus infection in turkey poults, a naturally infected wild turkey, and naturally infected bleeding heart doves caused severe myositis with intralesional megaloschizonts, muscle necrosis and lameness.2 Significant myositis was not found in any of our ten cases.Â
Descriptions of lesions virtually identical to those found in our cases have been reported in psittacine and nonpsittacine birds,12 with the designation of Haemosporozoa of undetermined taxonomic status. As described by Gardiner, this group of protozoal organisms is characterized by protozoal cysts within viscera and skeletal muscle without identification of gamonts or gametes in peripheral blood cytology.7 A recent report describing similar hepatic lesions in passerine and nonpasserine birds used PCR to confirm Haemoproteus as the etiologic agent.6 Interestingly, DNA sequence analysis of a conserved area of the cytochrome B gene reveals that the Haemoproteus sp. in the aforementioned cases is identical to the Haemoproteus in our cases. The presence of the same species of Haemoproteus in different orders of birds is a surprising finding, which challenges the previous designation of high host specificity of this protozoal disease.
Host sharing involving both Haemoproteus and Plasmodium was reported to occur between populations of migrating European birds and resident African birds,16 suggesting that these hemoparasites may be less host specific than previously believed. Host switching of protozoal hemoparasites into na_ve populations has been linked with changes in virulence and increased morbidity.1,3,14 These findings imply that host switching is a conceivable mechanism for Haemoproteus infection of birds from different localities assimilated into a novel environment, as is typical in a zoological setting. Although avian species from similar regions of the world are typically grouped together in a zoo, insect vectors can move between groups, and may play a role in transmission of infectious organisms. Interestingly, the majority of the birds affected (9 of 10) at our institution are from South America, excluding the bird from this case, the magnificent bird of paradise. In general, hemoprotozoa of neotropical birds are rare.15 Therefore, birds from this region of the world may have an increased susceptibility to hemoprotozoal infections. Sequence analysis of PCR products from our cases revealed that the sequences were identical and most consistent with Haemoproteus spp. from North American passerine birds. Thus, it is possible that infection with North American Haemoproteus in South American birds (abberrant hosts) may result in an aberrant parasitic form or a more prominent or virulent pre-erythrocytic form of the parasite. A similar example may be the introduction of new Plasmodium species to Hawaii, which resulted in high mortality rates and limited range habitation of native passerine species1, illustrating the impact engendered by introduction of a novel parasite to a naive population.Â
Iron stains confirmed the presence of hemosiderin within hepatocytes. Hemosiderosis is a common finding in many avian species, particularly in frugivorous or insectivorous birds. Birds of paradise are listed as a family within the order Passeriformes (Paradisaeidae) in which hepatic or multisystemic hemosiderosis is often found.10 Other commonly affected families include Ramphastidae and Sturnidae. Iron storage disease in these families may be primary or due to vulnerability to dietary overload, as opposed to secondary causes, including infection or inflammation.10 One must be careful to differentiate between hemosiderosis, meaning that there is excessive stainable iron in parenchymal or phagocytic cells, and hemochromatosis, meaning that the excess iron damages the cell, tissue or organ. In this case, although hemosiderin is widespread throughout the liver, no cellular damage or reactive changes attributable to the hemosiderin are apparent, which warrants a diagnosis of hemosiderosis.Â
JPC Diagnosis:
2. Liver: Hepatitis, portal, lymphoplasmacytic, multifocal, moderate.
3. Liver, hepatocytes: Hemosiderosis, diffuse, moderate.
Conference Comment:
Haemoproteus, Plasmodium, and Leucocytozoon gametocytes can all be found within the peripheral blood, although several differences exist among the three groups. In Leucocytozoon sp., gametocytes may also be found in leukocytes and will severely distort host cells.9 Mature Haemoproteus gametocytes within the erythrocytes of birds are located within the cytoplasm and partially encircle the nucleus without causing nuclear displacement (halter shape).6 Megaloschizonts are frequently present in tissues in cases of leucocytozoonosis and with infection of some species of Haemoproteus, but are not characteristic of Plasmodium sp.
References:
2. Atkinson CT, Greiner EC, Forrester DJ: Pre-erythrocytic development and associated host responses to Haemoproteus meleagridis (haemosporina: Haemoproteidae) in experimentally infected domestic turkeys. J Protozool 33(3):375-381, 1986
3. Bensch S, Stjernman M, Hasselquist D, Ostman O, Hansson B, Westerdahl H, Pinheiro RT: Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proceedings of the Royal Society of London, B: Biological Sciences 267:1583-1589, 2000
4. Burmudez, AJ: Miscellaneous and sporadic protozoal infections. In: Diseases of Poultry, ed. Saif YM, Barnes HJ, Glisson JR et al., 11th ed., pp 1014-15. Iowa State Press, Ames, IA,2003
5. Campbell TW: Common avian blood parasites. In: Avian hematology and Cytology, 2nd ed., pp. 30-31. Iowa State Press, Ames, IA, 1995
6. Campbell TW: Section III: Hematology of common non-domestic animals. In: Vetenirary Hematology and Clinical Biochemistry, ed. Thrall MA, pp. 245-248. Lippincott Williams & Wilkins, Philadelphia, PA, 2004
7. Ferrel ST, Snowden K, Marlar AB, Garner M, Lung NP: Fatal hemoprotozoal infections in multiple avian species in a zoological park. J Zoo Wildl Med 38(2):309-316, 2007
8. Gardiner CH, Fayer R, Dubey JP: Apicomplexa: Haemoproteus and Haemosporozoa of undetermined taxonomic status. In: An Atlas of Protozoan Parasites in Animal Tissues, 2nd ed., pp. 74-75. Armed Forces Institute of Pathology, American Registry of Pathology, Washington DC 20306, 1998
9. Greiner EC, Ritchie BW: Parasites. In: Avian Medicine: Principle and Applications, eds. Ritchie BW, Harrison GJ, Harrison LR, pp. 1019-1021. Wingers Publishing Inc., Lake Worth, FL, 1994
10. Lowenstine LJ and Munson, L: Iron Overload in the Animal Kingdom. In: Zoo and Wildlife Medicine, Current Therapy, eds. Fowler ME, Miller RE, 4th ed., pp. 260-265. WB Saunders Company, Philadelphia, PA, 1999
11. Macwhirter P: Passeriformes. In: Avian Medicine: Principle and Applications, eds. Ritchie BW, Harrison GJ, Harrison LR, p. 1197. Wingers Publishing Inc., Lake Worth, FL, 1994
12. Panigrahy B, Harmon BG, Grumbles LC: Hemorrhagic Disease in Canaries (Serinus canarius). Av Dis 28:536-541, 1984
13. Peirce, MA: Hematozoa. In: Avian Medicine, ed. Samour J, pp. 247-248. Mosby, Elsevier Science Limited, Philadelphia, PA, 2003
14. Schrenzel M.D, Maalouf GA, Keener LL, Gaffney PM: Molecular characterization of malarial parasites in captive passerine birds. J Parasitol 89:1025-33, 2003
15. Valkiunas G, Salaman P, Iezhova TA: Paucity of hematozoa in colombian birds. J Wildl Dis 39:445-448, 2003
16. Waldenstrom J, Bensch S, Kiboi S, Hasselquist D, Ottosson U: Cross-species infection of blood parasites between resident and migratory songbirds in Africa. Mol Ecol 11:1545-54, 2002