Signalment:
Twelve-year-old, male, dwarf rabbit, (
Oryctolagus cuniculus).Both testicles were submitted for histological examination because of
increasing testicular size over time.
Gross Description:
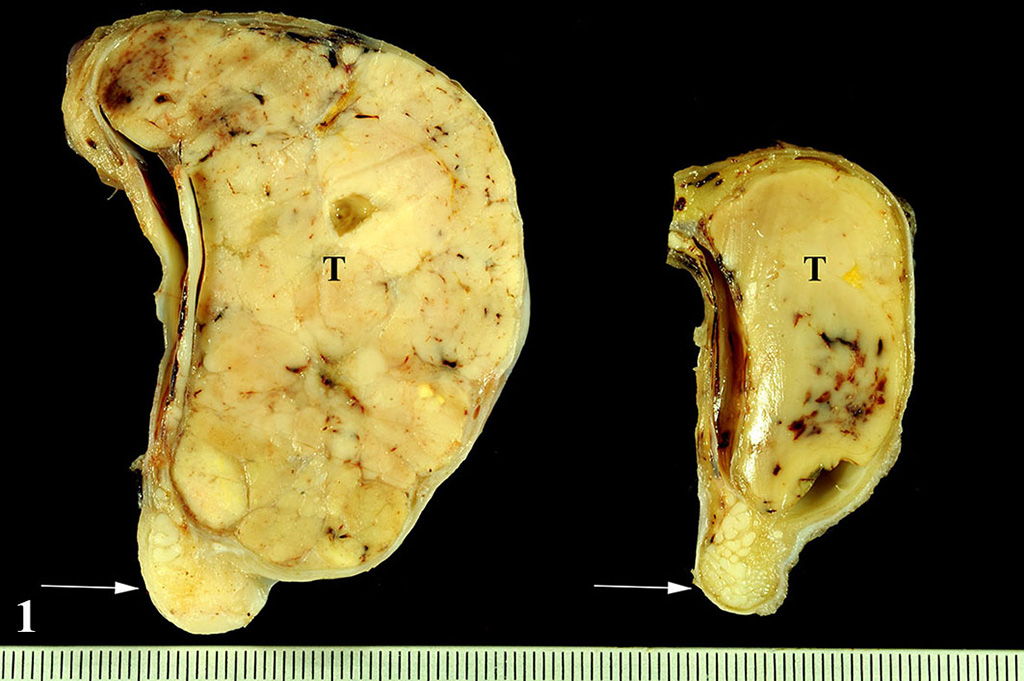
The formalin-fixed testicles measured 9 x 7 x 4 cm and 5
x 3 x 3 cm, respectively. The testicular parenchyma was almost completely
replaced bilaterally by multilobulated, solid masses with a greyish cut
surface. The mass was located on both sides within the testis and did not
extend beyond the tunica albuginea.
Histopathologic Description:
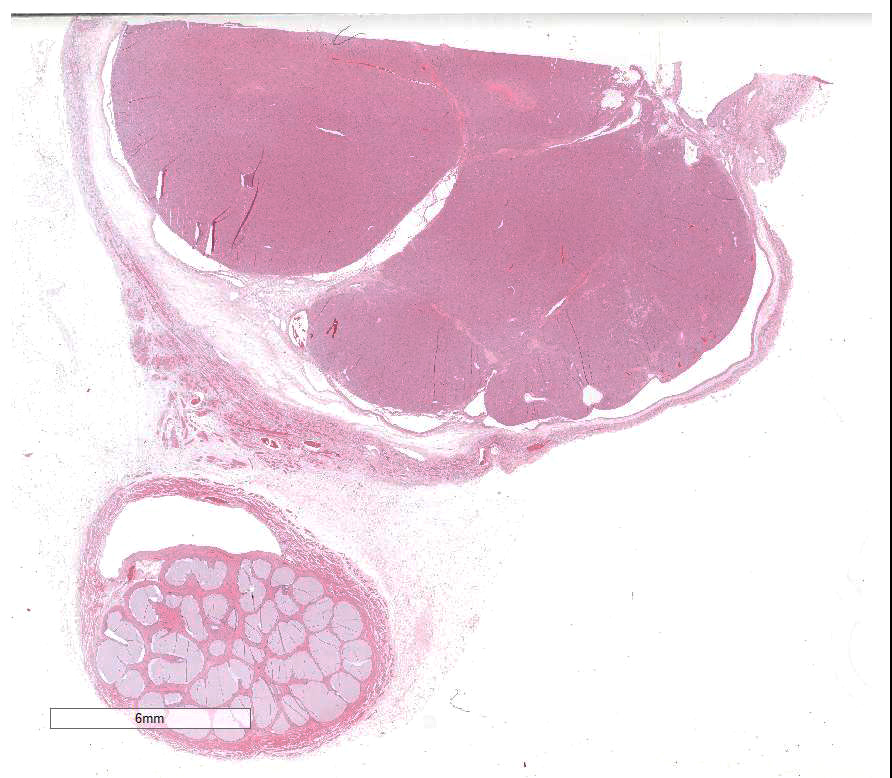
The slide contains parts of the testicle, epididymis and tunica
vaginalis. The majority of original testicular tissue is replaced by a
multilobular, well demarcated, non-encapsulated, expansive, moderately
cell-dense mass extending to the cut border on one side. Neoplastic cells are
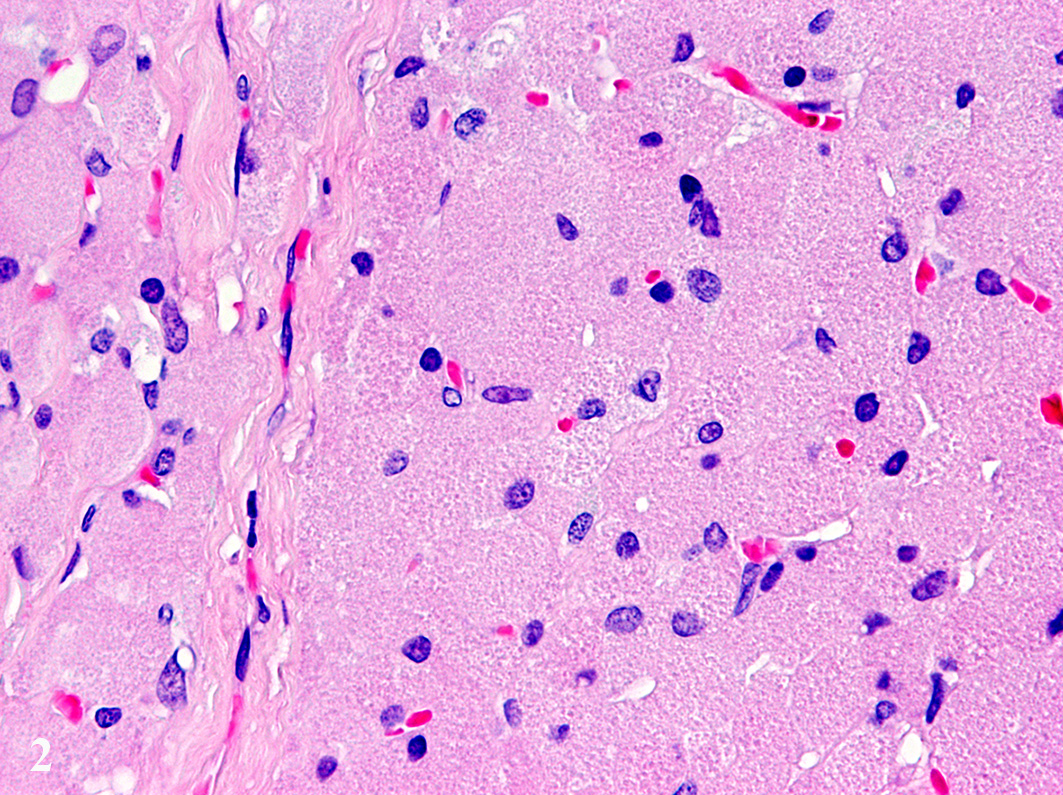
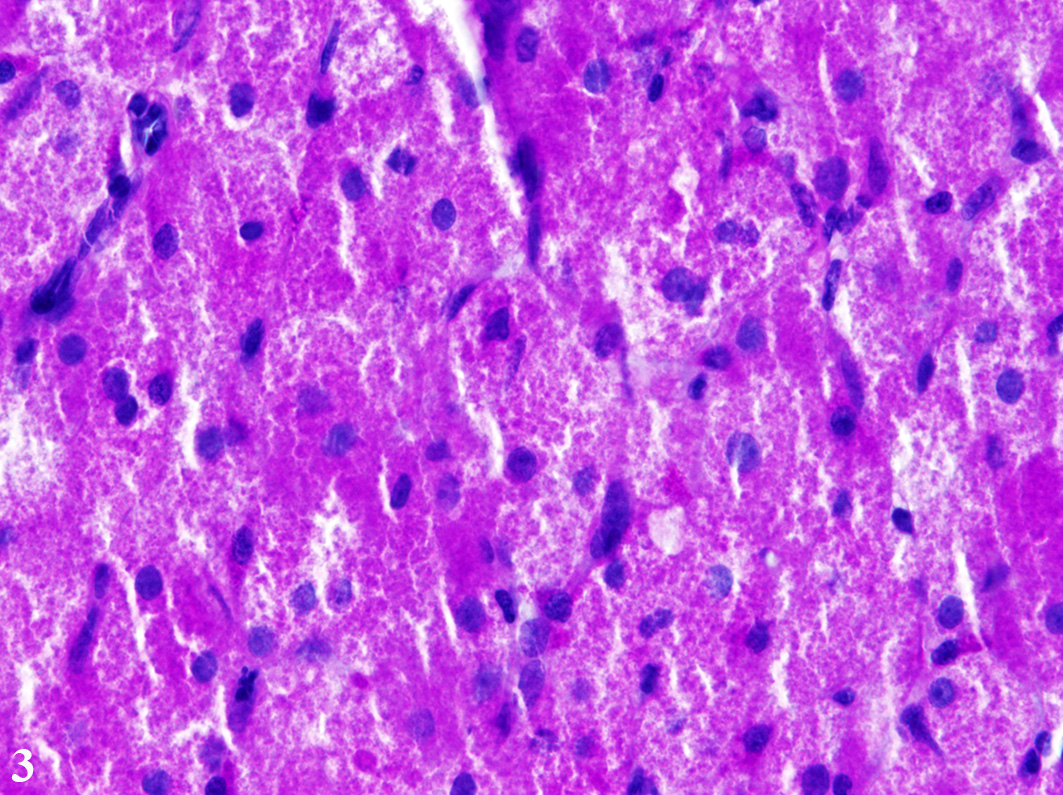
proliferated in solid fields supported by a small to moderate amount of
fibrovascular stroma. The cells measure up to 70 µm in diameter and display a
round to polygonal shape with an eccentrically located, 10 µm in diameter
large, round to oval nucleus with finely stippled heterochromatin and one distinct,
small, basophilic nucleolus. The abundant, eosinophilic, finely granular
cytoplasm is surrounded by indistinct cell borders. Tumor cells show mild
anisocytosis and anisokaryosis with a mitotic rate of 0-1 per high power field.
In the cytoplasm of the tumor cells, a high number of PAS and
PAS-diastase-resistant positive granules are present. These granules were also
shown by Luxol Fast Blue staining. Remaining seminiferous tubules are
compressed and lined by single Sertoli cells.
Multifocally
within the tunica vaginalis there are few inflammatory cells, mainly consisting
of plasma cells, lymphocytes, and fewer macrophages. In some slides
eosinophilic, homogeneous, acellular material is present within the tunica
vaginalis (edema). The epithelium of epididymal tubules is flattened and the
diameter of the tubules is severely increased (dilatation). Within the
epididymal tubules, no spermatozoa are present.
Morphologic Diagnosis:
Testicle: Granular cell tumor with
severe testicular atrophy, dilatation of epididymal tubules and mild, chronic,
multifocal, lymphohistiocytic and plasmacellular infiltration of the tunica
vaginalis with edema.
Lab Results:
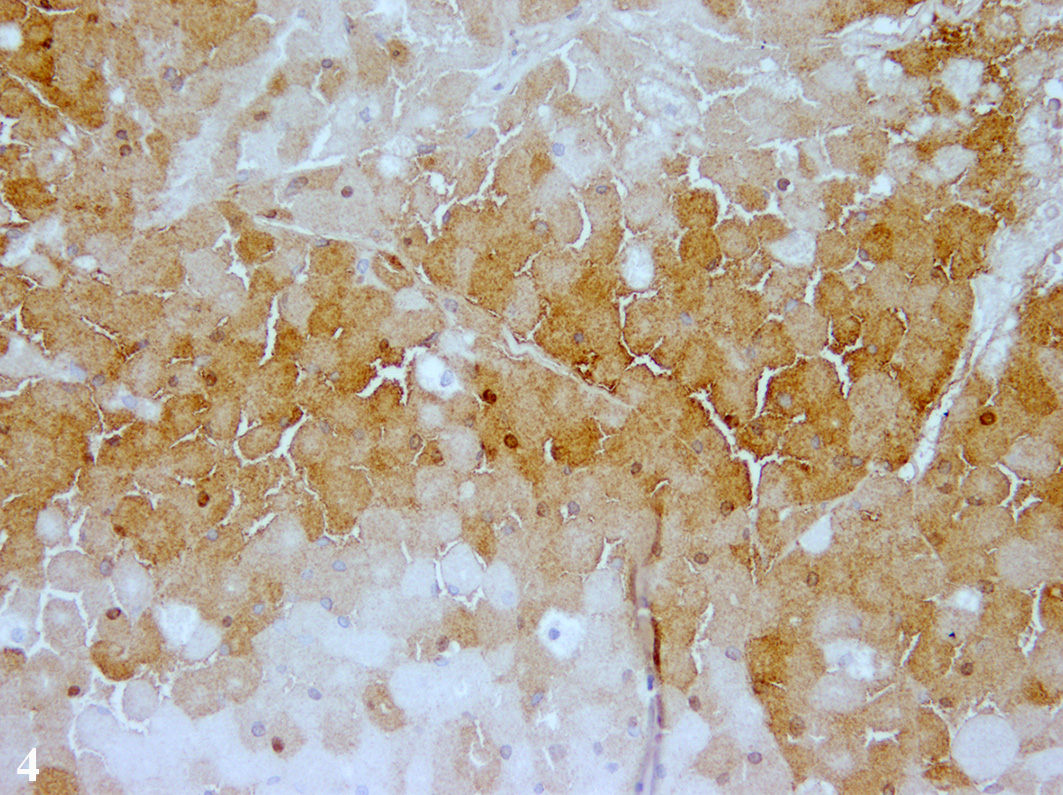
Immuno-histochemistry was applied using commercially available
antibodies. Tumor cells showed a diffuse immunolabelling for Melan-A and a
multifocal expression of neuron-specific enolase in about 40-50% of the tumor
cells. About 20-30% of the tumor cells displayed an immunolabelling for S-100
protein and single tumor cells expressed vimentin.
Neoplastic
cells were negative for cytokeratin, α-smooth muscle actin, glial
fibrillary acidic protein, and myelin basic protein. Samples
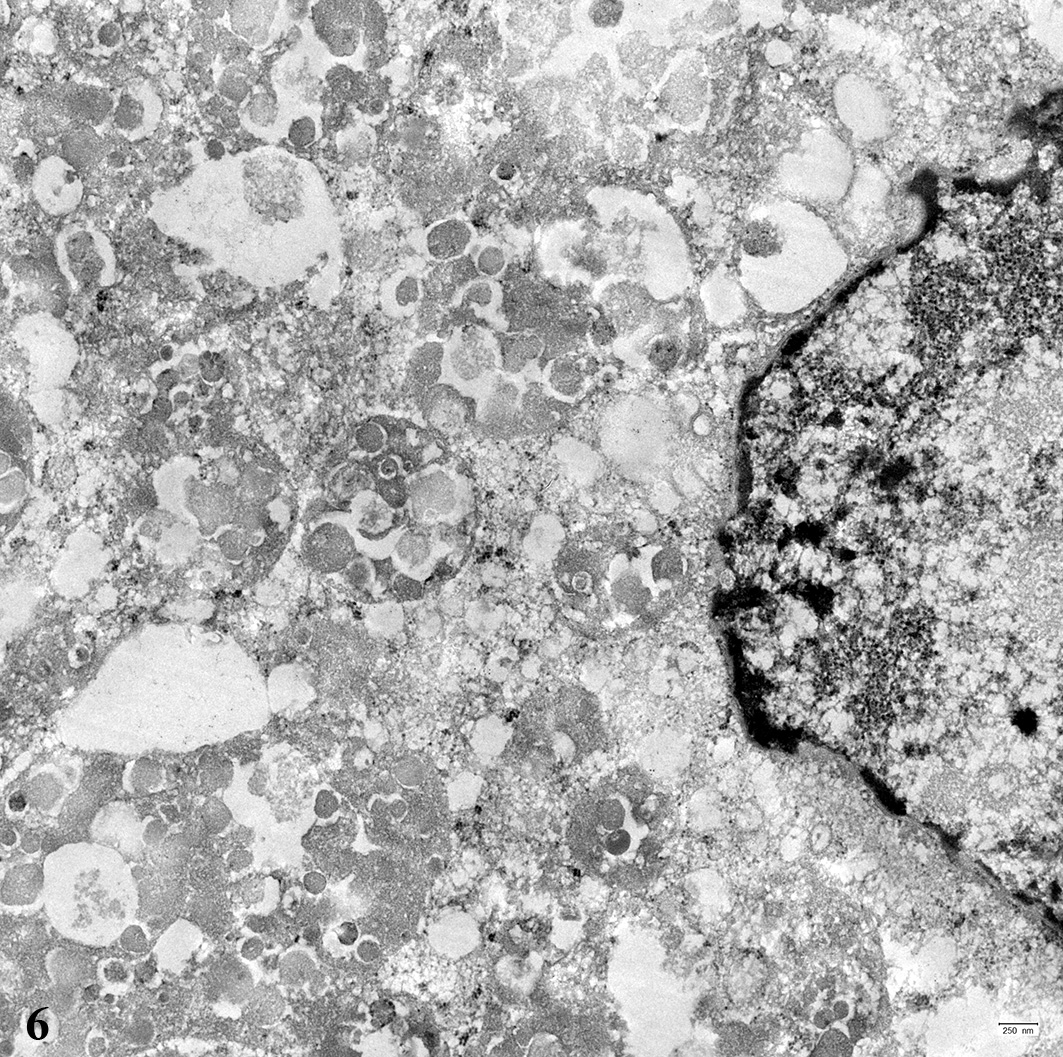
for transmission electron micro-scopy were processed by direct pop-off
technique from the HE- stained slide. The cytoplasm of the tumor cells was
filled with numerous round, membrane-bound structures measuring about 500 to
1000 nm in diameter. These structures contained several membrane-bound,
moderately electron-dense, round structures, measuring about 50 to 150 nm in
diameter.
Condition:
Granular cell tumor
Contributor Comment:
Testicular tumors in rabbits represent a rarely described
entity. Mainly adult individuals are affected and interstitial cell tumors are
most commonly reported.
6,7,19
Granular
cell tumors occur rarely in domestic and pet animals and have been reported in
different species including horses, dogs, cats, guinea pigs, and rats.
2,6 In
14 In horses,
granular cell tumors represent the most common primary neoplasm of the lung.
2,9
In dogs and rarely in cats, they occur in the oral cavity.
2,11 In
rats, a meningeal localization has been described.
18 A variant of
meningiomas, termed granular cell meningioma, has been reported in dogs located
at the cerebral convexity, neurohypophysis, and spinal nerve roots.
1
Genital
involvement of granular cell tumors has been reported in cats
affecting the vulva.
5,6 Within the right atrium of a dog a granular
cell tumor, also termed myo-blastoma, has been described exhibiting typical
cytoplasmic granules.
13 In guinea pigs, cutaneous granular cell
tumors have been reported.
17
The histogenetic origin of granular cell tumors remains unknown,
but they are thought to derive from Schwann cells or related cells due to their
histological, immunohistological and electron micro-scopic characteristics.
7,11
The immuno-reactivity of granular cells to antibodies specific for S-100
protein, Melan-A, and neuron-specific enolase supports the potential neuro-ectodermal
origin.
6,7
Furthermore,
ultrastructurally numerous round cytoplasmic structures are found surrounded by
a membrane with a diameter of about 500 to 1000 nm containing round
electron-dense structures, measuring about 50 to 150 nm in diameter. These
findings are similar to reports about the ultrastructure of equine pulmonary
granular cell tumors.
12,15 The membrane-bound structures tend to
contain fragments of mitochondria or other organelles and are interpreted as
secondary lysosomes.
The
majority of granular cell tumors are reported to be benign, except one case in
a cat, showing recurrence after excision and a high degree of pleomorphism with
a high mitotic rate.
11 Granular cell tumors in cats generally tend
to be more anaplastic.
11
As a differential diagnosis interstitial cell or Leydig cell tumor
has to be considered because it shares histologic features with the granular
cell tumor, e. g. eosinophilic granules within the cytoplasm.
6 In
hematoxylin-eosin stained slides they are almost indistinguishable. However,
testicular interstitial cell tumors lack PAS-positive and diastase-resistant
cytoplasmic granules as well as Luxol Fast Blue stainable granules that are
indicative for a granular cell tumor.
7,11 Furthermore, granular cell
tumors express neuron-specific enolase, S-100 protein, and vimentin to a
variable extent
6,7,19 as was shown in the present case.
Additionally, transmission electron microscopy is a suitable tool to
demonstrate the typical membrane-bound granules reported for granular cell
tumors, leading to their name.
6,12<
JPC Diagnosis:
Testis: Granular cell tumor, dwarf
rabbit, (
Oryctolagus cuniculus).
Conference Comment:
The contributor provides an excellent summary of the major
features of granular cell tumors (GCT). GCTs are histologically characterized by
proliferating uniform polygonal neoplastic cells that contain abundant
eosinophilic granules in their cytoplasm and a round eccentrically placed
nucleus.
2 Conference participants discussed differential diagnoses
for neoplasms with abundant granular eosinophilic cytoplasm to
include rhabdomyomas, oncocytomas, balloon cell
melanoma, and interstitial cell tumors. In addition to the histochemical and
immunohistochemical stains mentioned by the contributor, transmission electron
microscopy remains the best way to differentiate these histologically similar
neoplasms. Ultrastructurally, oncocytomas and rhabdomyomas both contain large numbers of
mitochondria which explain the abundant acidophilic granular appearance
histologically.
2,3,6 In balloon cell
melanoma, electron microscopically reveals numerous heterogeneous melanosomes
within the cytoplasm. Interstitial cell tumors (also known as Leydig cell
tumors) have an abundance of smooth endoplasmic reticulum and well-developed
mitochondria with tubular and vesicular cristae. In GCT, the granules are thought to be composed of numerous
membrane-bound secondary lysosomes.
2,3,6
In a 2015
Veterinary Pathology article, Suzuki et al. demonstrates
that in canine lingual GCT, the cytoplasmic granules are positive for LC3, p62,
NBR1, and ubiquitin. LC3 is localized on the membranes of autophagosomes and is
a potent marker of autophagy. In addition, LC3, p62, and NBR1 are all required
for production of the autophagosome.
14 This suggests that the
cytoplasmic granules found in canine lingual GCT cells are autophagolysosomes
which are a subset of secondary lysosomes that result
from the fusion of a primary lysosome with a phagosome containing cytoplasmic
cellular constituents that are to be digested.
14
Recently,
it has been suggested that autophagy might play a suppressive role in the
initiation stages of a neoplasm by maintaining genomic stability and inducing
cell senescence and autophagic death; but conversely plays a maintaining role
in tumor growth in the later stages of tumorigenesis by supplying metabolic
substrate, limiting oxidative stress, and maintaining cancer stem cell
population.
8
Due to near
diffuse effacement and compression of the normal testicular architecture
and distortion from diffuse ductular ectasia in the adjacent epididymis,
conference participants had some trouble identifying the tissue of origin in
this section. The key to identification of the tissue is dependent on a close
investigation of the epididymis. The epididymis
is a tightly coiled mass of thin tubules that carries sperm from the testes to
the ductus deferens in the male reproductive system. In this case, the
epididymal tubules are markedly dilated, lined by a single layer of attenuated
cuboidal epithelium and contain an abundant amount of amphophilic inspissated
proteinaceous material. Some participants noted that scattered throughout the
lumen are very small numbers of degenerate spermatozoa thus identifying the
tissue as epididymis with adjacent testis and tunica vaginalis.
References:
1. Cantile
C, Youssef S. Nervous system. In: Maxie MG, ed.
Jubb, Kennedy, and Palmer's
Pathology of Domestic Animals. 6
th ed. Vol. 1. St. Louis,
Missouri: Elsevier; 2016:396397.
2. Caswell
JL, Williams KJ. Respiratory System. In: Maxie MG, ed.
Jubb, Kennedy, and
Palmer's Pathology of Domestic Animals. 6
th ed. Vol. 2. St.
Louis, Missouri: Elsevier; 2016:482,498.
3. Goldblatt
PJ, Gunning WT. Ultrastructure of the interstitial cells of Leydig, stimulated
and unstimulated.
Ann Clin Lab Sci. 1985; 15(6):441-450.
4. Goldschmidt MH, Dunstan RW, Stannard AA,
Tscharner CV, et al.
Histological classification of epithelial and
melanocytic tumors of the skin of domestic animals. Vol III. 2nd
series. Washington D.C.: Armed Forces Institute of
Pathology. 1998:38-39.
5. Han
X, Yu L, Yang S, Zheng J. Primary neuroendocrine tumor of the testis: a study
of clinicopathological features. Int J Clin Exp Pathol.
2014; 7(4):17711776.
6. Irizarry-Rovira
AR, Lennox AM, Ramos-Vara JA. Granular cell tumor in the testis of a rabbit:
cytologic, histologic, immunohistochemical, and electron microscopic
characterization. Vet Pathol. 2008; 45(1):7377.
7. Kelley
LC, Hill JE, Hafner S, Wortham KJ. Spontaneous equine pulmonary granular cell
tumors: morphologic, histochemical, and immunohistochemical characterization. Vet
Pathol. 1995 ;32(2):101106.
8. Lu
SZ, Harrison-Findik DD. Autophagy and cancer. World J Biol Chem. 2013;
4)3):64-70.
9. Maratea KA, Ramos-Vara JA, Corriveau LA, Miller MA. Testicular
interstitial cell tumor and gynecomastia in a rabbit. Vet Pathol.
2007;44(4):513517.
10. Ohnesorge B, Gehlen
H, Wohlsein P. Transendoscopic electrosurgery of an equine pulmonary granular
cell tumor. Vet Surg. 2002; 31(4):375378.
11. Patnaik AK.
Histologic and immunohistochemical studies of granular cell tumors in seven
dogs, three cats, one horse, and one bird. Vet Pathol. 1993;
30(2):176185.
12. Parker GA, Novilla
MN, Brown AC,Flor WJ, Stedham MA. Granular cell tumor (myoblastoma) in the lung
of a horse. J Comp Pathol. 1979;8 9(3):421430.
13. Robinson WF, Robinson
NA. Cardiovascular System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's
Pathology of Domestic Animals. 6th ed. Vol. 3. St. Louis,
Missouri: Elsevier; 2016:5253.
14. Suzuki S, Uchida K,
et al. The origin and role of autophagy in formation of cytoplasmic granules in
canine lingual granular cell tumors. Vet Pathol. 2015; 52(3):456-464.
15. Turk MAM, Breeze RG.
Histochemical and ultrastructural features of an equine pulmonary granular cell
tumor (myoblastoma). J Comp Pathol. 1981; 91(4):478481.
16. Wilkerson
MJ, Dolce K, DeBey BM, Heeb H, et al. Metastatic Balloon Cell Melanoma in
a dog. Vet Clin Pathol. 2003; 32(1):31-6.
17. Willmes A, de
Leuw N, Wohlsein P. Fallbericht: Kutaner Granular-zelltumor bei einem
Meerschweinchen. Prakt Tierarzt. 2013; 94:198205.
18. Wright JA,
Goonetilleke UR, Waghe M, Stewart M, Carlile A. Comparison of a human granular
cell tumour (myoblastoma) with granular cell tumours (meningiomas) of the rat
meninges--an immunohistological and ultrastructural study. J Comp Pathol.
1990;103(2):191198.
19. Zwicker GM, Killinger
JM. Interstitial cell tumors in a young adult New Zealand white rabbit. Toxicol
Pathol. 1985; 13(3):232235.