Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 17
5 February, 2020
CASE I: 1235813-006 (JPC 4117518).
Signalment: Three year old, male cynomolgus macaque (Macaca fascicularis) from China
History: This animal was found dead on day 109 of a 6 month routine general toxicity study. There were no reported clinical signs. Routine infectious agent screens and three consecutive tuberculosis skin tests were negative with the exception of measles which was positive after a live vaccine.
Gross Pathology There were moderate, focal to multifocal, tan foci on the liver and lung with moderate swelling of the mediastinum. Skin discolorations with abrasions / scabs were present on the ventral abdomen and right forelimb.
Laboratory results: The tan foci were PCR positive for Mycobacterium tuberculosis group and culture yielded slow growing acid fast bacilli identified as Mycobacterium tuberculosis.
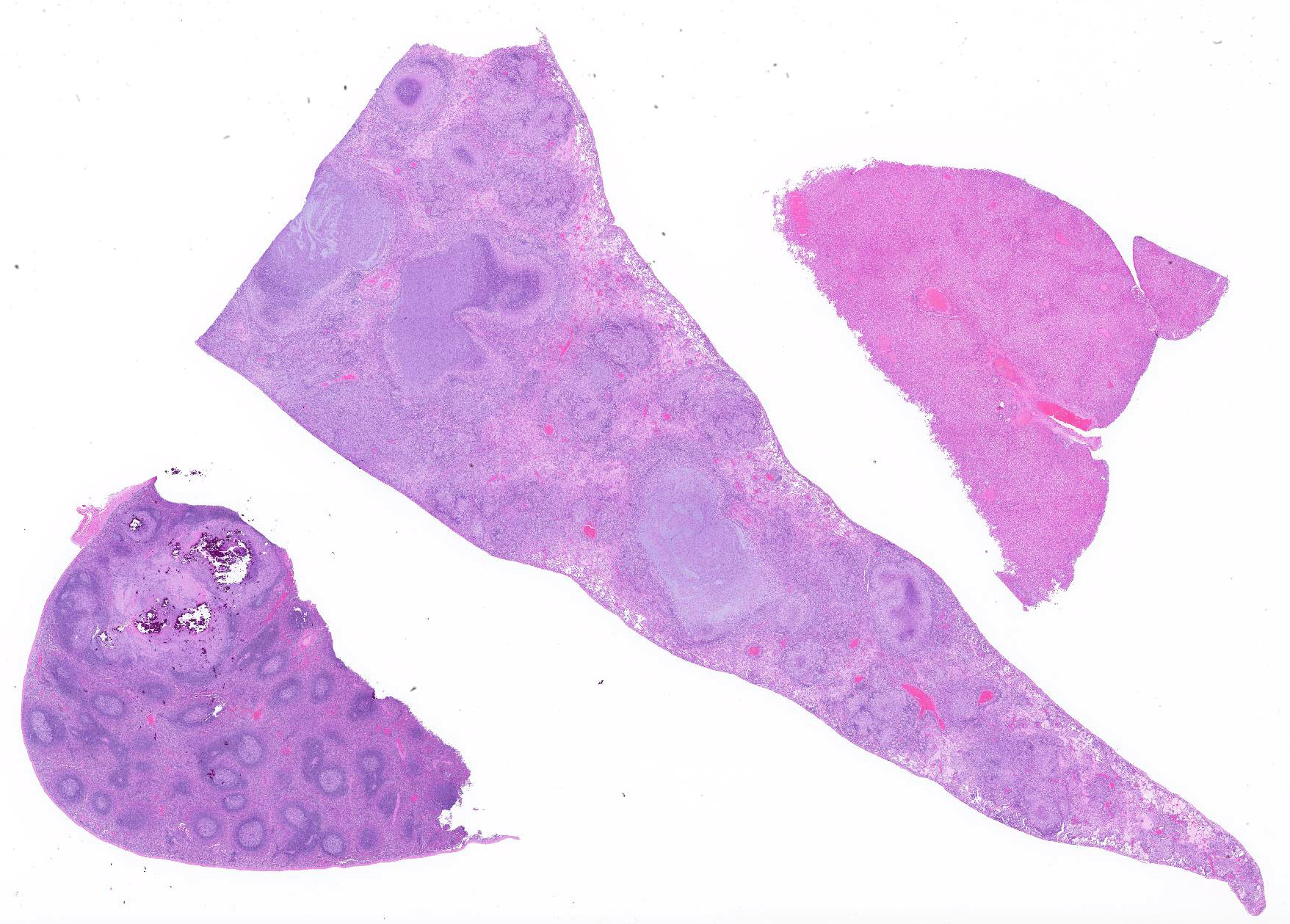
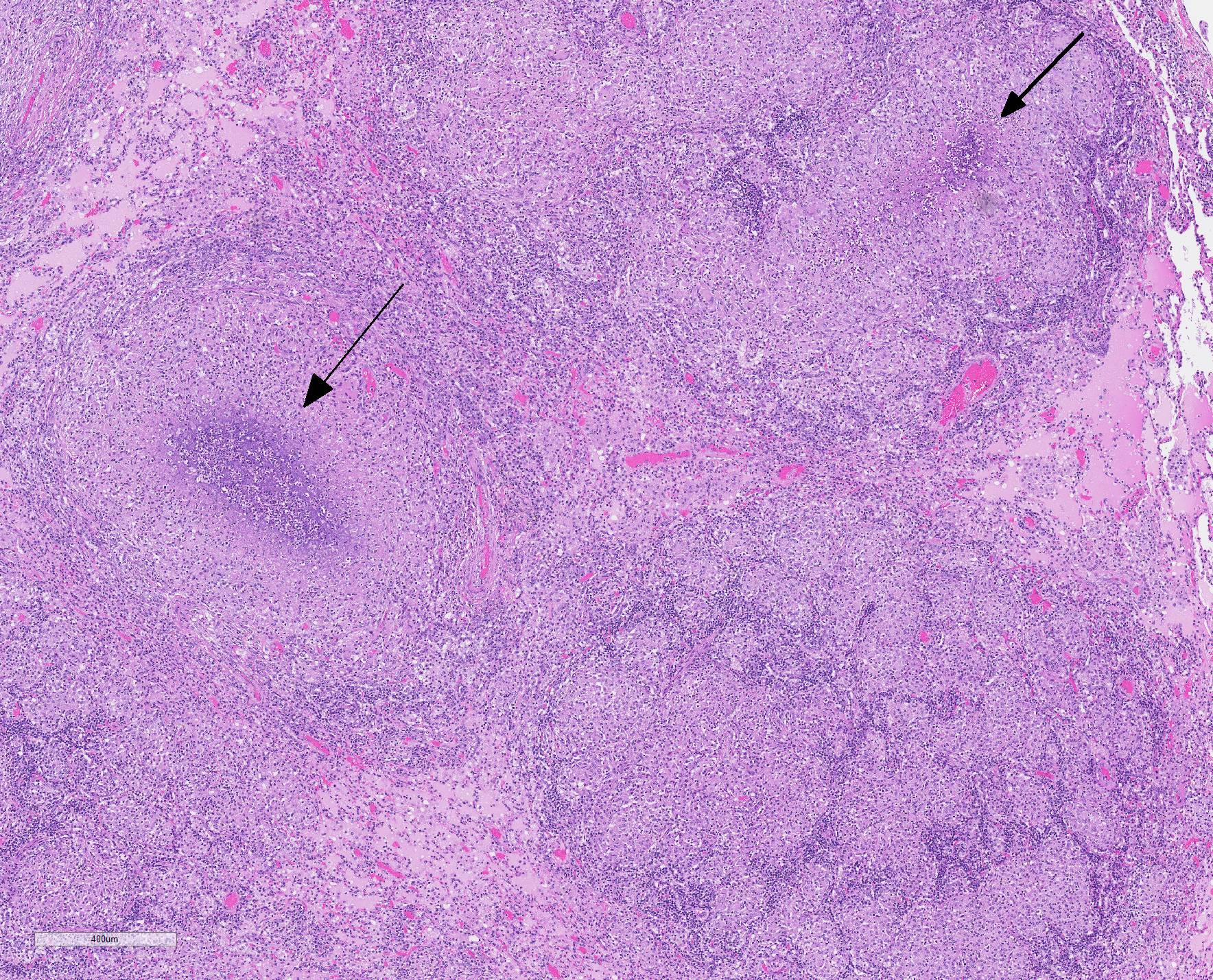
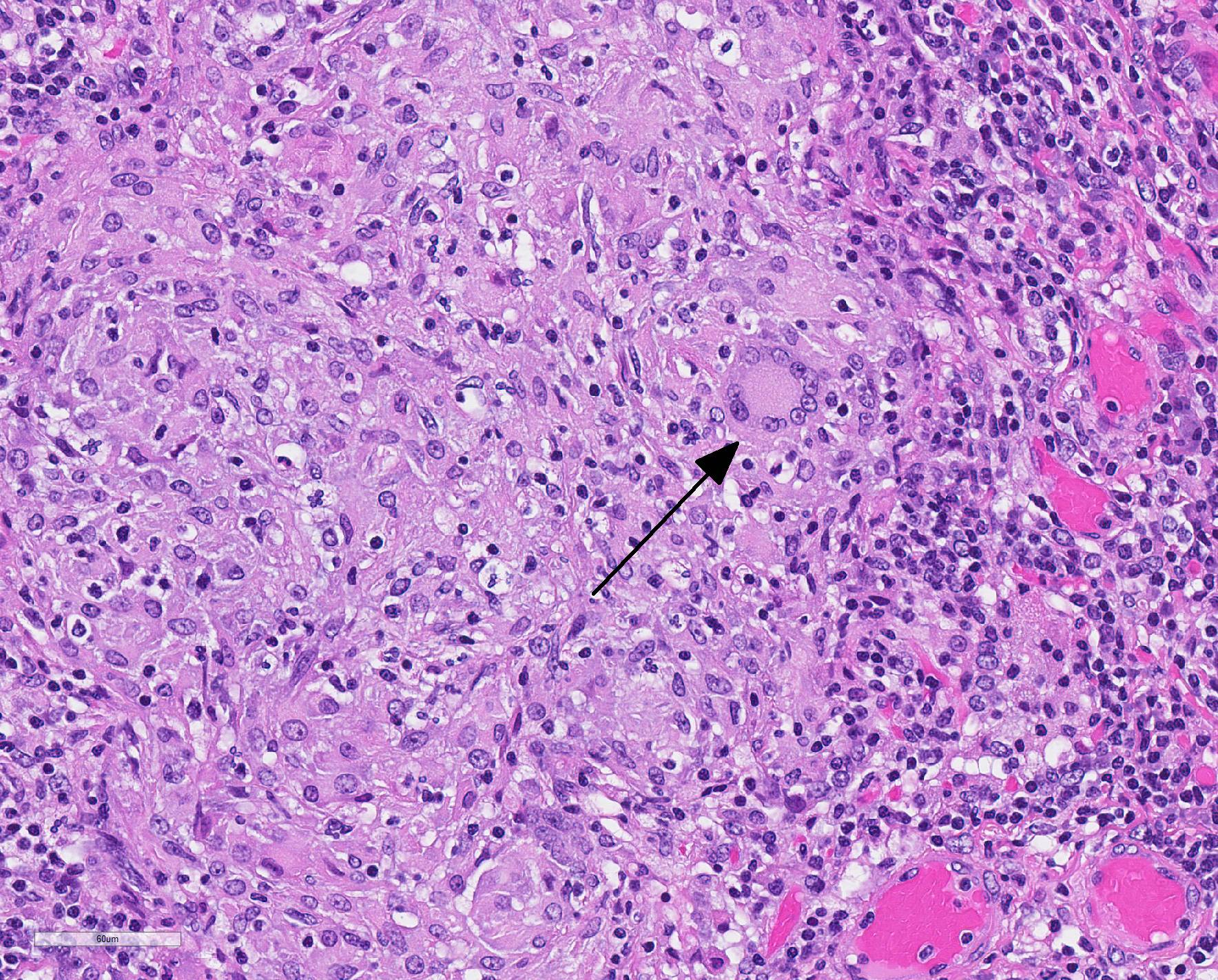
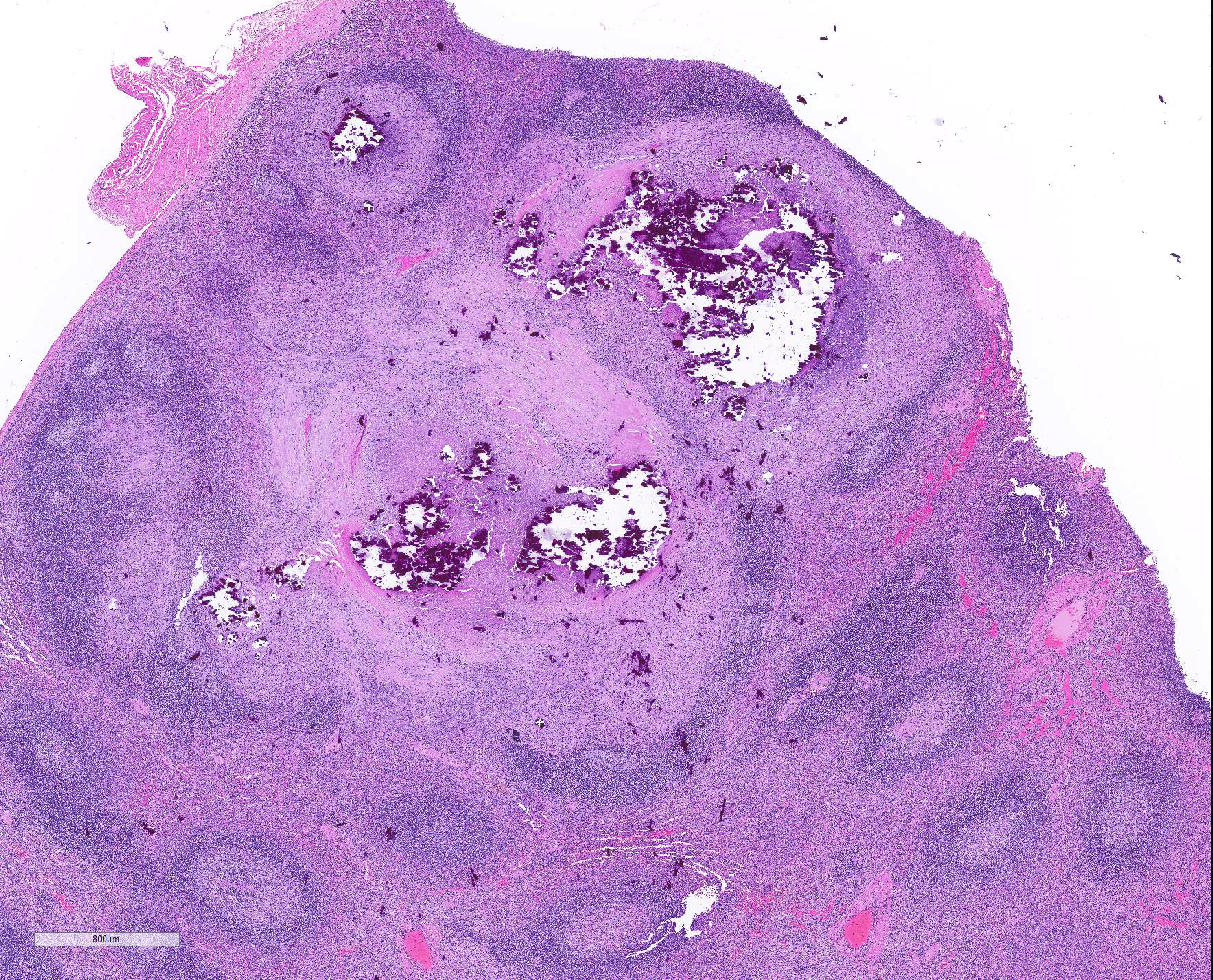
Microscopic Description: Throughout approximately 90% of examined lung there was multifocal to coalescing granulomatous inflammation often forming discrete granulomas with central eosinophilic flocculent debris (caseating necrosis) with or without basophilic granular mineralization. This central area was surrounded by epithelioid macrophages, multinucleated giant cells, a rim of lymphocytes and variable fibroblasts/fibrosis. Similar solitary discrete granulomas were seen in the spleen, liver, and mediastinum (mediastinum not submitted). There was an increase in number and prominence of germinal centers in the spleen (follicular hyperplasia) as well as frequent multifocal type 2 pneumocyte hyperplasia in the lung.
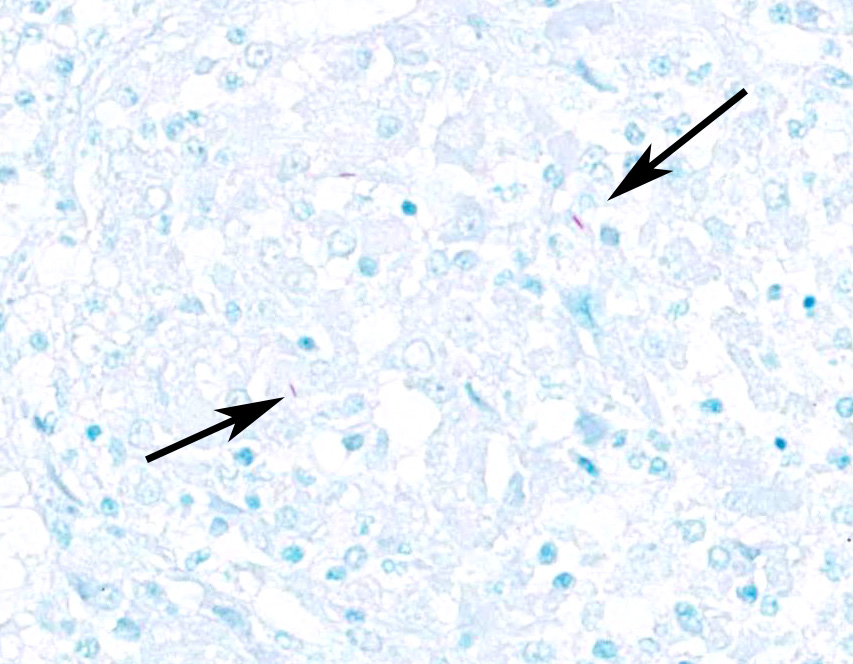
Ziehl-Nielsen acid fast staining of the lung revealed rare acid fast bacilli within the cytoplasm of macrophages or multinucleated giant cells, and/or free within the necrotic/mineralized areas.
Contributor Morphologic Diagnosis:
Granulomatous pneumonia, severe, diffuse with focal hepatic and splenic granulomas and scant acid-fast bacilli.
Contributor Comment: Mycobacterium tuberculosis (M.tb) is a cosmopolitan organism that infects approximately 1/3 of the world?s human population and is a leading cause of death due to infectious disease. It is also an increasing public health concern due to 1) emerging multidrug resistant and extensively drug resistant M.tb and 2) the increasing risk of death in HIV infected people.20 Tuberculosis was considered the cause of 40% of all HIV-related deaths in 2016.20 Roughly 10% of people infected with M.tb go on to develop active contagious disease with the remaining individuals either clearing the bacteria (approximately 10%) or harboring latent disease. These latent carriers create a reservoir of tuberculosis which can reactivate during times of stress or immune suppression and disseminate M.tb to naïve populations of humans20 or spread the disease to susceptible animals (reverse zoonosis).13
In an NHP necropsy, M.tb often presents as tan or creamy white nodules (granulomas), predominantly within the lung, but also with systemic involvement. Other common causes of tan nodules or granulomas within the lung includes foreign body (kaolin), protozoan (Toxoplasma gondii), bacterial (Nocardia sp), mycotic (Coccidioides immitis, Coccidioidoes posadasii, Histoplasma capsulatum, Blastomyces dermatitidis, and Cryptococcus neoformans) and parasitic organisms (Pneumonyssus simicola (acariasis), Taenia cysts, Echinococcus cysts, Mesocestoides tetrathyridia, Spirometra spargana, and Filaroides and Filariopsis nematodiasis), Histology can distinguish between these diagnoses based on the appearance of the organisms.11
M.tb and mycobacteria in general are unusual among bacteria due to the high lipid content and relative thickness of their cell wall compared to gram-positive and gram-negative bacteria. The thick mycobacterial cell wall is relatively impermeable, does not take up crystal violet (Gram stain variable), and is responsible for the acid fast staining properties of mycobacteria. Immunologically, the lipid component of the mycobacterial cell wall is particularly effective at recruiting an immune response and has been commonly used as an adjuvant in vaccines to recruit a desired robust immune response to antigens of interest. The protein (antigen) and lipid components together during mycobacterial infection result in sensitization of the animal to tuberculosis proteins. Sensitized animals then react to purified mycobacterial protein derivative (PPD) in a skin test with a Type IV hypersensitivity reaction resulting in a cell mediated immune reaction after 2-3 days. Non-responders, or animals that fail to demonstrate a positive response to the PPD skin test in the face of mycobacterial infection (as seen in this monkey) are thought to be due to immunologic anergy.3 Anergy is a failure of the animal to produce a Type IV delayed type hypersensitivity reaction upon PPD exposure. The exact cause of anergy in M.tb infection is unknown; although, in general, it is a lack of response of cell mediated immunity due to tyrosine kinase blockage and reduced IL-2 receptor signaling, among other mechanisms.17 In M.tb infection a weak cell-mediated immune response (high IL-4) is thought to be associated with anergy.7
Another feature of the mycobacterial cell wall is its role in allowing the bacteria to evade the host immune system. In part, the success of M.tb is due to the highly infectious nature of the bacteria. It is thought that as few as 10 bacteria can cause disease.18 Once within the host, mycobacteria are engulfed by macrophages into phagosomes. Under normal circumstances within a macrophage, phagosomes mature and fuse with lysosomes exposing the bacteria to destructive chemicals and lytic enzymes leading to bacterial death. Mycobacteria have evolved mechanisms to avoid phagosome-lysosome fusion and survive within macrophages. For example, components of the mycobacterial cell wall interrupt the normal traffic of the phagosome to the lysosome by preventing recruitment of key molecules in phagosomal maturation such as the Rab5 to Rab7 conversion.1 Phagosome maturation arrest in M.tb is thought to involve specific cell surface receptors (i.e. mannose receptor) and mycobacterial cell wall components (i.e. lipoarabinomannan and SapM enzyme)5. Ultimately, whether the mycobacteria are destroyed or remain active or latent within the host depends upon the infectious dose, the host immune response, and the environment of the infection.3 A host response characterized by a Th2 cytokine response (IL-4) is associated with mycobacteria infection and Th1 cytokine response (IL-12, TNF-α, and IFN-γ) is associated with bacterial clearance.1
There are many mycobacteria that cause significant human and animal diseases. The histologic features of these diseases range from structured granulomas (often associated with few bacteria/paucibacillary infection) to more diffuse macrophage infiltration (often associated with numerous bacteria/multibacillary infection). Granulomas, or tubercles, have four histologic features: 1) central caseous necrosis with or without mineralization, 2) surrounded by epithelioid macrophages and Langhans multinucleated giant cells, 3) surrounded by lymphocytes with or without neutrophils and peripheral fibrosis, 4) tubercles contain rare acid fast bacilli which are found within macrophages, multinucleated giant cells, and/or the caseous core. In paucibacillary mycobacterial diseases, gross observations are crucial to identifying areas to look for these rare bacilli as bacteria are not typically found outside of the structured granulomas.3. Multibacillary infection is more variable with few to sheets of infiltrating macrophages containing few to numerous acid fast bacilli. Leprosy (Mycobacterium leprae) is centered on nerves and can be paucibacillary and granulomatous or multibacillary and diffuse depending upon the strength of the cell mediated immune response with a stronger cell mediated response associated with the paucibacillary infection.7 Common mycobacterial species involved in disease are listed in Table A.
|
Table A3 |
|||
|
Mycobacteria |
Species Affected |
Systems involved and/or Gross appearance |
Typical histologic appearance |
|
M. tuberculosis |
humans, NHPs, elephants, psittacine birds (zoonosis and reverse zoonosis)
exotic ungulates, marine mammals, carnivores14, dogs, cats, pigs, cattle
Potential reservoir species: humans |
Granulomas ? lung and systemic (i.e. liver, spleen, etc.) |
granulomas |
|
M bovis |
cattle, dogs/cats, humans, cervids, swine, horses
deer, elk, bison, buffalo, goats, camels, llamas, swine, elephants, rhinoceroses, dogs, foxes, cats, mink, badgers, humans, NHPs, pigs, goats, warthogs, cats, mustelids, humans
Potential reservoir species: badgers, brush tailed possums, swamp buffalo, buffalo, bison, various species of deer |
cattle: granulomas ? lung and systemic (lymph nodes, gastrointestinal tract, etc)
dogs and cats: skin nodules or thickening/inflammation
humans: pulmonary granulomas or gastrointestinal inflammation
cervids: pulmonary granulomas, superficial lymphadenitis with draining tracts (DDx: Fusobacterium necrophorum
horses: Gastrointestinal thickening/ granulomas
swine: systemic granulomas |
granulomas
dogs and cats: unstructured pyogranulomatous inflammation of the skin dissecting along facial planes. (Note: central necrosis and paucibacillary help to distinguish from feline leprosy); often more granulation tissue with few scattered macrophages. |
|
M avium subspecies avium and hominissuis6 |
birds > swine, cattle, horses, sheep, NHPs, humans |
birds: thin body condition with intestinal and hepatic > systemic granulomas (lungs, air sacs, spleen, bone marrow and skin)
humans: lymphadenitis, granulomas ? lung and/or systemic |
granulomas |
|
M. avium paratuberculosis |
cattle, goats, sheep |
muscle atrophy/wasting; thickened mucosa of the ileum, large intestine, enlarged draining nodes; intimal plaques/mineral in thoracic aorta
small ruminants: granulomas (enteric or vascular/lymphatic) |
multibacillary ? unstructured granulomatous enteritis with villus atrophy
sheep: lepromatous neuritis reported |
|
M.microti18 |
voles, humans |
voles: gastrointestinal granulomas
humans: pulmonary granulomas |
granulomas |
|
M.africanum4 |
humans |
Pulmonary granulomas |
granulomas |
|
M.avium intracellularae complex (MAC) |
dogs, cats |
atypical mycobacterial skin infection - singe to multiple cutaneous and subcutaneous nodules, plaques, macules or diffuse swelling In cats: thickened, hardened tissue in the inguinal fat |
multibacillary unstructured pyogranulomatous dermatitis and panniculitis. Can resemble feline leprosy. Occasionally fibroplasia may be marked and the lesion may appear as a spindle cell neoplasm |
|
M. marinum10 |
fish
humans |
fish: systemic disease (potential route of infection skin trauma or gastrointestinal tract)
humans: skin granulomas > pulmonary granulomas |
granulomas |
|
M. kansasii |
humans |
cervical lymphadenitis; pulmonary granulomas |
granulomas |
|
M.scrofulaceum15 |
humans |
cervical lymphadenitis ?scrofula? |
granulomas |
|
saprophytic Mycobacteria (M. smegmatis, M. fortuitum, M. chelonae8, M. phlei, M. thermoresistible, M. xenopi) |
dogs/cats |
Focal thickened skin / inflammation |
atypical mycobacterial skin infection - Similar to MAC although paucibacillary and clear vacuoles surrounded by neutrophils and peripheral epithelioid macrophages. Organisms can be found within the clear vacuoles |
|
M. leprae9 |
armadillos, sooty mangabeys, chimpanzees, cynomolgus macaques, red squirrels |
Multifocal to coalescing granulomatous dermatitis with loss of sensation/body parts on face, hands, and feet. |
granulomas (tuberculoid) to unstructured (lepromatoid) granulomatous inflammation centered on nerves |
|
M lepraemurium |
rodents, cats |
often head and limb cutaneous or subcutaneous nodules with or without alopecia or ulceration, often 1-3 year old cats |
granulomatous dermatitis and/or panniculitis ? large, pale, foamy macrophages; tuberculoid (organized granulomas) to lepromatous (sheets of cells); nerve involvement is only sporadic (distinct from human leprosy |
|
Mycobacterium? |
Cats |
Feline tuberculosis syndrome in adult cats with firm skin nodules with draining tracts |
pyogranulomatous dermatitis and panniculitis |
|
Mycobacterium? |
Dogs |
canine leproid granuloma syndrome ? firm painless nodules mainly on the head and dorsal pinnae of short coated breeds |
acid fast bacteria identified, but not cultured; histo description unavailable |
Contributing Institution:
MPI Research; a Charles River company
https:///www.crl.com
https://www.mpiresearch.com
JPC Diagnosis:
1. Lung: Pneumonia, bronchointerstitial, necrogranulomatous, multifocal to coalescing, severe, with Langhans multinucleated giant cells, mineralization, and edema.
2. Spleen: Splenitis, necrogranulomatous, multifocal to coalescing, severe, with mineralization.
JPC Comment: The contributor has provided an excellent review of infection by M tuberculosis, (which has also been a popular submission in non-human primates as well as various models over the years).
The very early history of Mtb is still conjecture, but early strains may have infected hominids up to three million years ao with modern strains originating some 15,000-20,000 years ago. Egyptian mummies have demonstrated skeletal deformitis reminiscent of "Pott&146;s disease", and are even present in Egyptian art. The first written documents about TB appeared in India and Chinica dating back to 3000 to 2000 years ago.2
TB
was well known and referred to as phthisis in ancient Greece. Isocrates
first suggested the contagious nature of "the king?s evil". The Romans
recognized the fist symptomatology of TB and recommended fresh air, milk, and
sea voyages as treatment2.
Pulmonary TB was well known in Middle Ages Europe, with "scrofula", a form of TB affecting
the cervical nodes, described as a new clinical form. Before the first
surgical interventions in the removal of scrofula, the touch of English or
French royalty was considered a cure (and continued in France until 1825).2
The
true infectious nature of "phthisis" and "consumption" was expounded upon by
English physician Benjamin Martin, and the disease was first called
"tuberculosis" Johan Schonlein in the mid 19th century. The
industrial revolution and poorly ventilated and overcrowded workplaces and
housing with poor sanitation resulting in a fluorishing of TB, with up to 15%
of workers dying of tuberculosis in 1838-1839 in England alone. The anemic
pllor of the afflicted resulted in another name ? "the white plague". The
"white plague" reached a zenith in the mid-1800s, resulting in almost one in
four deaths in Europe and North America2.
The 1800s also brought a new and revised view of tuberculosis, with numerous
renowned clinicians connecting the dots between miliary disseminated disease,
tubercules, consolidation, pleurisy and pulmonary cavitation. In 1843, German
physician Philipp Klencke was able to reproduce the human and bovine disease n
rabbits. In 1850, the "sanatorium cure" was described for the first time by
Hermann Berhmer, a botany student suffering from TB himself, and was used for
the next 100 years as a mainstay of treatment for TB patients.2
In 1882, Robert Koch identified, isolated, and for the first time visualized
the tubercle bacillus of Mycobacterium tuberculosis in a number of
manifestations of tuberculosis, including pulmonary, scofulaceous,
extra-pulmonary, and meningeal lesions. Following on his work, Paul Ehrlich
developed alternate methods of staining, which in the hands of Franz Ziehl and
Friedrich Neelson, became the first acid alcohol method for demnstrating the
bacillus in tissue.12
In
1895, Wilhelm Konrad von Rontgen discovered the use of X-rays and became the
first physician to visualize and detect TB lesions in the lungs; X-rays were an
inexpensive method of diagnostic screening together with the tuberculin skin
sensitivity test until the 1950s. In 1890 Koch himself developed a
concentrated filtrate of cultured tubercle bacilli which he called "tuberculin"
or "Koch?s lymph" which he believed could actually cure the disease. However,
when injected, it cause violnt ractions, high fever, expansion of pre-existing
lesions, and a severe reaction at the site, which he noted on self-inoculation.
While it never did cure the disease, it was noted that infected individuals
would develop "allergy" upon injection, resulting in an effected diagnostic
method. The tuberculin and its method of use in the skin continued to be
refined until the development of tuberculin purified protein defivative (PPD)
by American biochemists Florence Seibert and Esmond Long in 1930, which was
able to also detect latent subclinical infections.12
Vaccination for TB over the years has been problematic at worst, ineffective
most of the time, and today is used only in aras with a high prevalence of
childhood disease and in people in the military and healthcare with a high risk
of exposure. The most successful advance in vaccination came in 1905 by two
French bacteriologists, Albert Calmette and Camille Guerin at the Pasteur Institute,
who developed a live attenuated vaccine from a non-virulent stratin. Over
100,000 children were vaccinated with this strain in the 1930s across Europe.
Unfortunately 250 children were vaccinated with a BCG vaccine accidentally
contaminated with a virulent strain in Lubeck Germany; 73 died and 135
developed clinical tuberculosis. Childhood vacination continued in post-WWII
Europe and Asia to combat a resurgence of TB, but ultimately was discontined in
the 1970s, when strict control programs were considered sufficient to control
the spread.12
In 1993, the World Health organization declared TB a global emergency, and in 1995 defined the directly observed treatment, short-course strategy (DOTS) control policy, which has decreased the global incidence of TB by 1.5% each year, and overall mortality from TB has declined 22% from 200-2015. With new and effective anti-tuberculosis chemotherapeutic agens, the strategy of early diagnosis and targeted therapy is now considered to have the best cost/benefit ratio for treating this worldwide disease.12
The
moderator and attendees reviewed a number of genpath concepts regarding
mycobacterial infections, a number of of species and concepts regarding
mycobacterial infections in a variety of species, and some diseases that might
grossly cause nodules in the lungs of non-human primates. She mentioned the
current theory of aortic mineralization in cattle with severe granulomatous
disease as being the result of production of 1,25-cholecaliferol by the large
numbers of macrophages in this disease and hypercalcemia.
References:
1. Ashenafi S, Aderaye G, Bekele A, Zewdie M, Aseffa G, Hoang ATN, Carow B, Habtamu M, Wijkander M, Rottenberg M, Aseffa A, Andersson J, Svensson M, Brighenti S. Progression of Clinical Tuberculosis is Associated with a Th2 Immune Response Signature in Combination with Elevated Levels of SOCS 3. 2014. Clin Immunol.151:84-99.
2. Barberis I, Bragazzi NL, Galluzo L, Martini M. The history of tuberculosis: from the first historical records to the isolation of Koch?s bacillus. J Prev Med Hyg 2017; 58:E9-12.
3. Caswell JL, Williams KJ. Respiratory System. In: Jubb, Kennedy, and Palmer?s Pathology of Domestic Animals. Vol 2. 5th ed. Editor: Maxie MG. Philadelphia, PA: Elsevier; 2007: 606-610
4. de Jong BC, Antonio M, Gagneux S. Mycobacterium marinum ? Review of an Important Cause of Human Tuberculosis in West Africa. 2010. PLOS Negl Trop Dis. 4(9):1-9
5. Deretic V, Singh S, Master S, Harris J, Roberts E, Kyel G, Lee HH, Vergne I. Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defence mechanism. 2006. Cell Microbio 8(5):719-727
6. Dhama K, Mahendran M, Tiwari R, Singh SD, Kumar D, Singh S, Sawant PM. Tuberculosis in Birds: Insights into the Mycobacterium avium Infections. 2011. Vet Med Intern. 1-14
7. Ginn PE, Mansell JEKL, Rakich PM. Skin and appendages. In: Jubb, Kennedy, and Palmer?s Pathology of Domestic Animals. Vol 1. 5th ed. Editor: Maxie MG. Philadelphia, PA: Elsevier; 2007: 688-689
8. Greer LL, Strandberg JD, Whitaker BR. Mycobacterium chelonae Osteoarthritis in a Kemp?s Ridley Sea Turtle (Lepidochelys kempii). 2003. J Wild Dis 39(3): 736-741
9. Honap TP, Pfister LA, Housman G, Mills S, Tarara RP, Suzuki K, Cuozzo FP, Sauther ML, Rosenberg MS, Stone AC. Mycobacterium leprae Genomes from Naturally Infected Nonhuman Primates. 2018. PLOS Negl Trop Dis. 12(1):1-17
10. Lescenko P, Matlova L, Dvorska L, Bartos M, Vavra O, Navratil S, Novotny L, Pavlik I. Mycobacterial Infection in Aquarium Fish. 2003. Vet Med ?Czech (3):71-78
11. Lowenstine LJ, Osborn KG. Respiratory System Diseases of Nonhuman Primates. In: Nonhuman Primates in Biomedical Research: Diseases. Vol 2. Editors: Abee CR, Mansfield K, Tardif S, Morris T. London, UK: Elsevier; 2012: 413-48.
12. Martini M, Besozzi G, Barberis I. The never-ending story of the fight against tuberculosis: from Koch?s bacillus to global control programs. J Prev Med Hyg 2018:E241-247.
13. Messenger AM, Barnes AN, Gray GC. Reverse Zoonotic Disease Transmission (Zooanthroponosis): A Systematic Review of Seldom-Documented Human Biological Threats to Animals. 2014. PLOS One 9(2):1-9
14. Montali RJ, Mikota SK, Cheng LI. Mycobacterium tuberculosis in Zoo and Wildlife Species. 2001. Rev Sci Tech 20(1):291-303
15. Moulis G, Martin-Blondel G. Scrofula, the King?s Evil. 2012. CMAJ 184(9).
16. Pieters J. Mycobacterium tuberculosis and the macrophage: Maintaining a Balance. 2008. Cell Host & Microbe 3:399-407
17. Schwartz RH. T cell Anergy. 2003. Annu Rev Immunol 21:305-334
18. Smith NH, Crawshaw T, Parry J, Birtles RJ. Mycobacterium microti: More Diverse Than Previously Thought. 2009. J Clin Microbiol. 47(8):2551-2559
19. Yan L, Cui H, Xiao H, Zhang Q. Anergic Pulmonary Tuberculosis is Associated with Contraction of the Vd2+T cell Population, Apoptosis, and Enhanced Inhibitory Cytokine Production. 2013. PLOS One 8(8):1-8
20. http://www.who.int/news-room/fact-sheets/detail/tuberculosis