Signalment:
2.5 month old, intact female, Yak (
Bos
grunniens) This yak was bottle-raised from birth and
housed in a small pen in the backyard, abutting a building. The yak had an
approximately two week history of pica and a one week history of decreased
appetite and lethargy, which progressed to loss of a suckle response. The
animal developed a fever (104F) and was initially treated with antibiotics and
tube feedings of electrolytes and milk at the farm but was referred for
suspected laryngeal or pharyngeal trauma based on difficulty tubing and reflux
of milk through the nose. On presentation to the referral hospital, the yak was
tachypneic (112 rpm) with harsh lung sounds bilaterally and decreased lung
sounds ventrally. The yak was markedly azotemic (results below) and was started
on intravenous fluids and other supportive care. Thoracic radiographs supported
cranioventral pneumonia and a trans-tracheal wash was performed (results below)
and antibiotics were initiated. The yak's azotemia did not improve with fluid
therapy and the yak was producing minimal urine. The yak developed ascites and
a total of 4L was removed via abdominocentesis on the two days preceding
euthanasia. The yak did not suckle at any time during hospitalization. The yak
was euthanized 7 days after admission to the referral hospital.
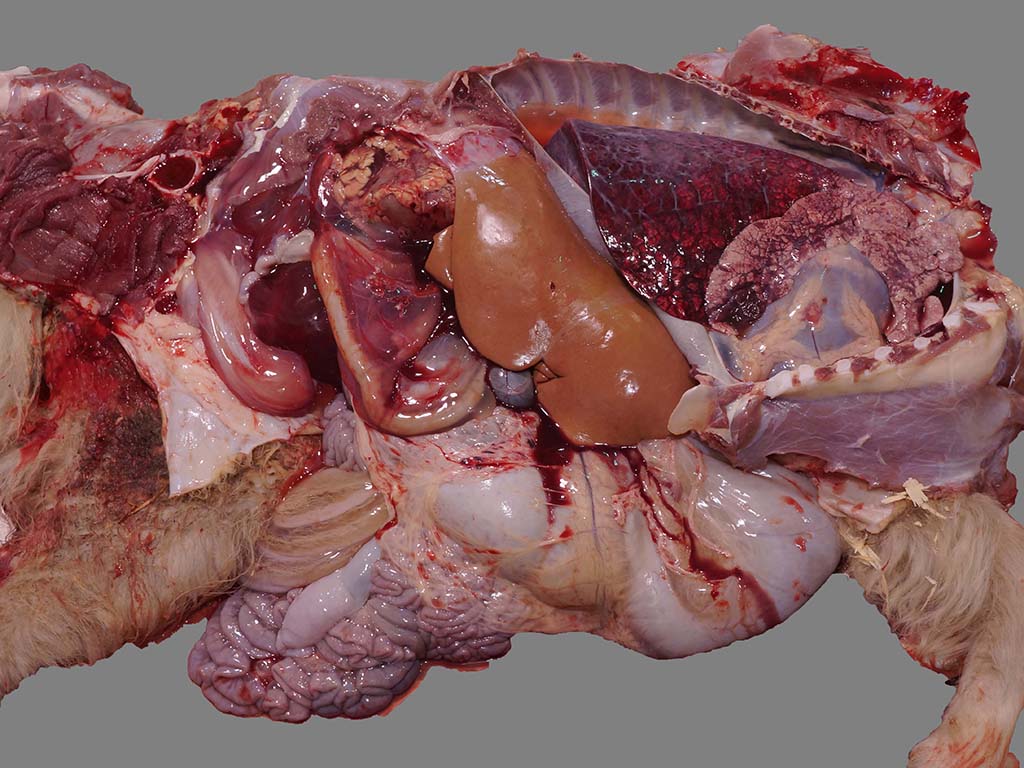
Gross Description:
The abdominal cavity contained 2.5 L of
translucent, pale pink to yellow, watery fluid and there was marked perirenal
edema with mild to moderate hemorrhage, more pronounced surrounding the left
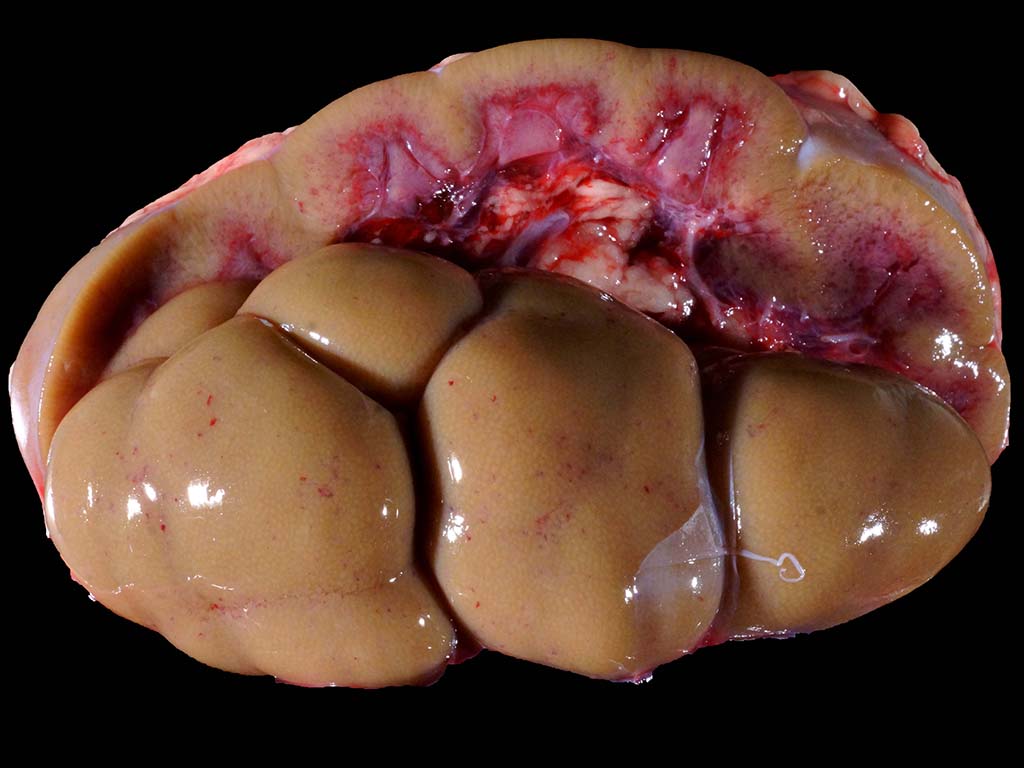
kidney. The kidneys were diffusely pale brown and slightly swollen, with small
pinpoint foci of hemorrhage in the cortex, reddening of the corticomedullary
junction, and hemorrhage and edema in the adipose tissue of the renal pelvis.
There was also marked mesocolonic edema and mild to moderate generalized
subcutaneous edema. There were no lesions in the esophagus, pharynx or larynx.
The rumen contained a moderate amount of gray opaque liquid and small chips of
gray paint. The liver was diffusely pale brown. The thoracic cavity contained
0.3 L of fluid and the pericardium contained 0.1 L of fluid, similar to the
abdominal fluid. The lungs were dark red caudally and mottled pink and dark red
cranially with diffuse interstitial edema and multiple dark red, firm areas of
consolidated lung
bilaterally in the cranioventral lung fields, consistent with aspiration
pneumonia (foreign material present on histopathology).
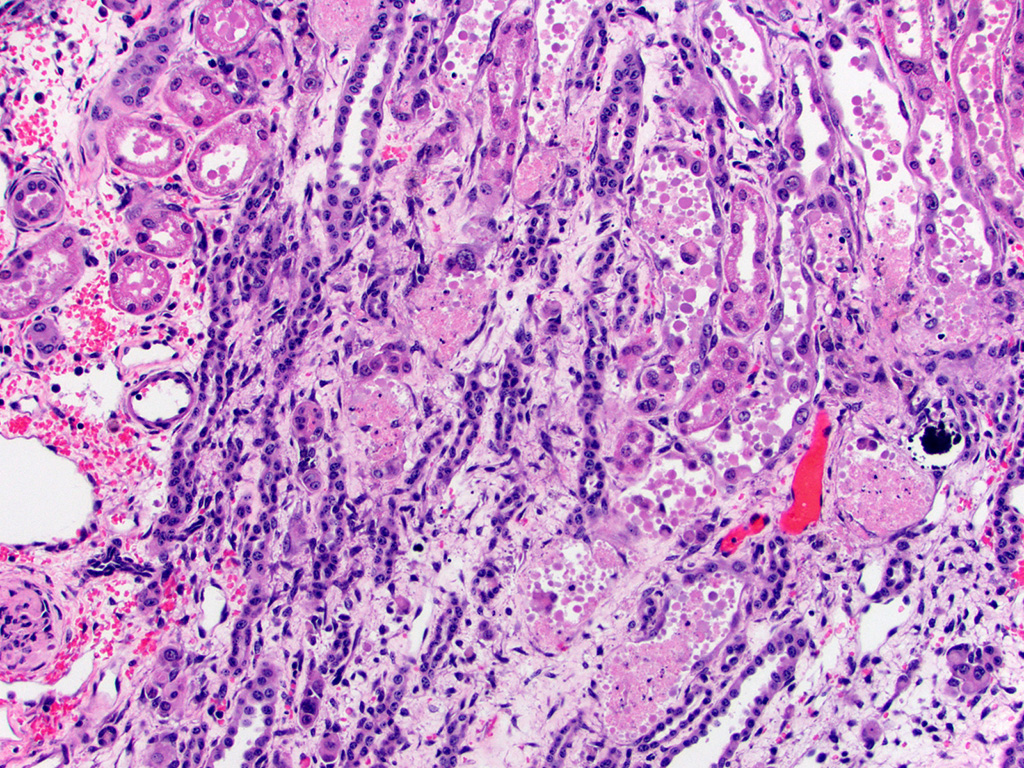
Histopathologic Description:
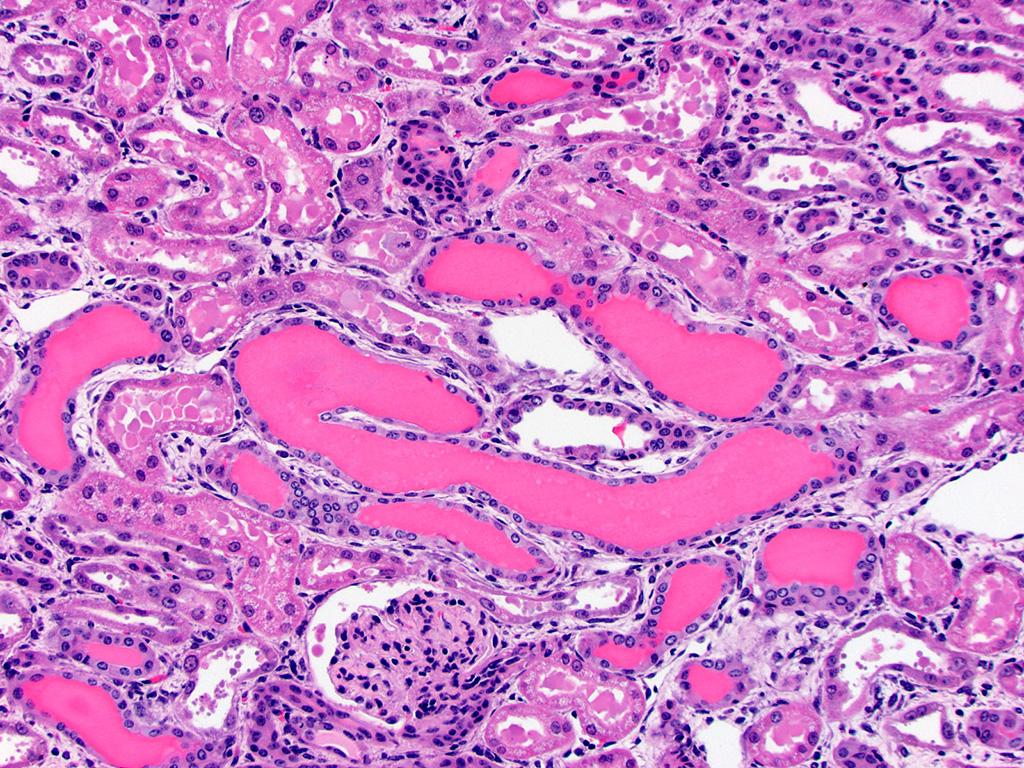
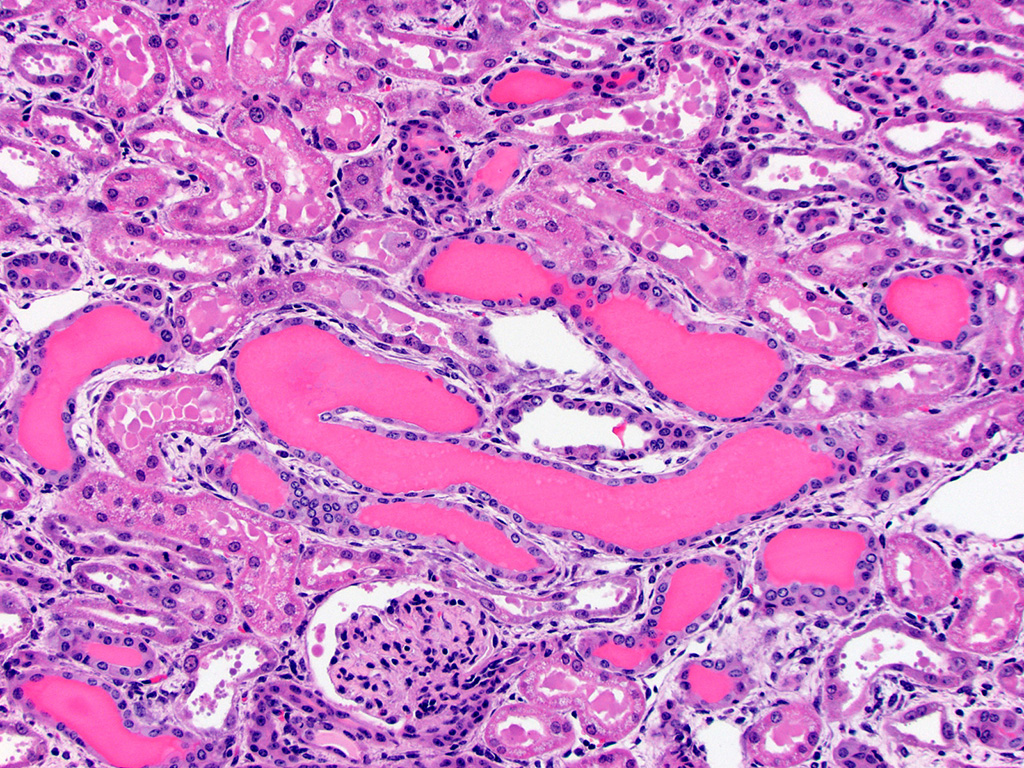
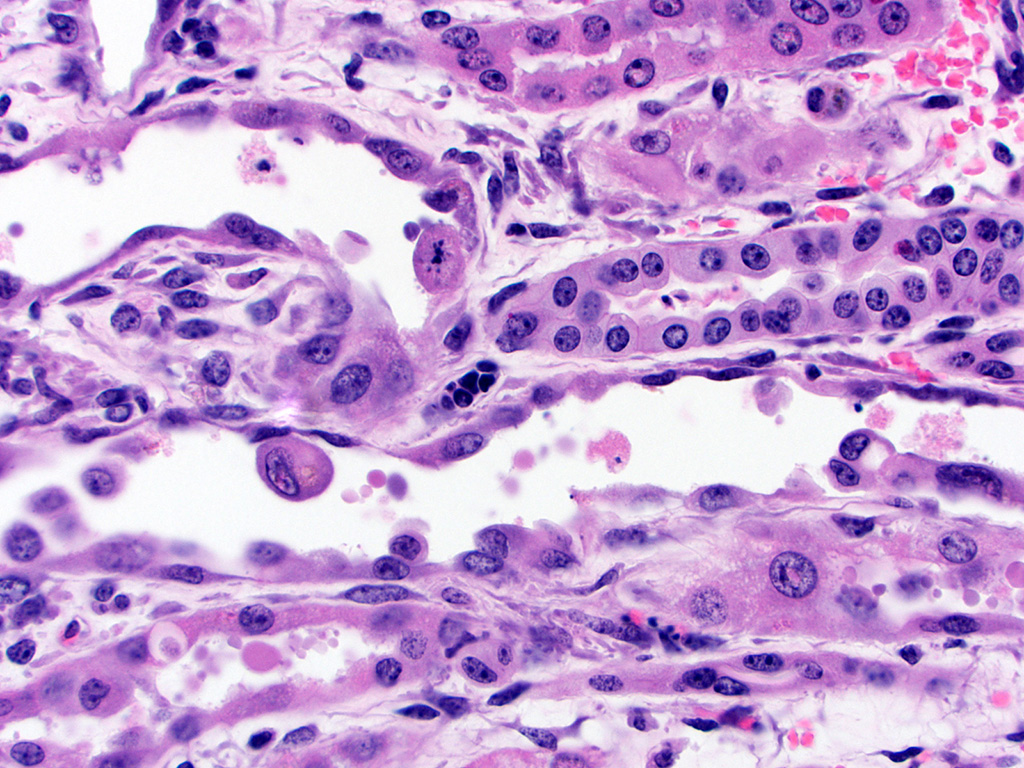
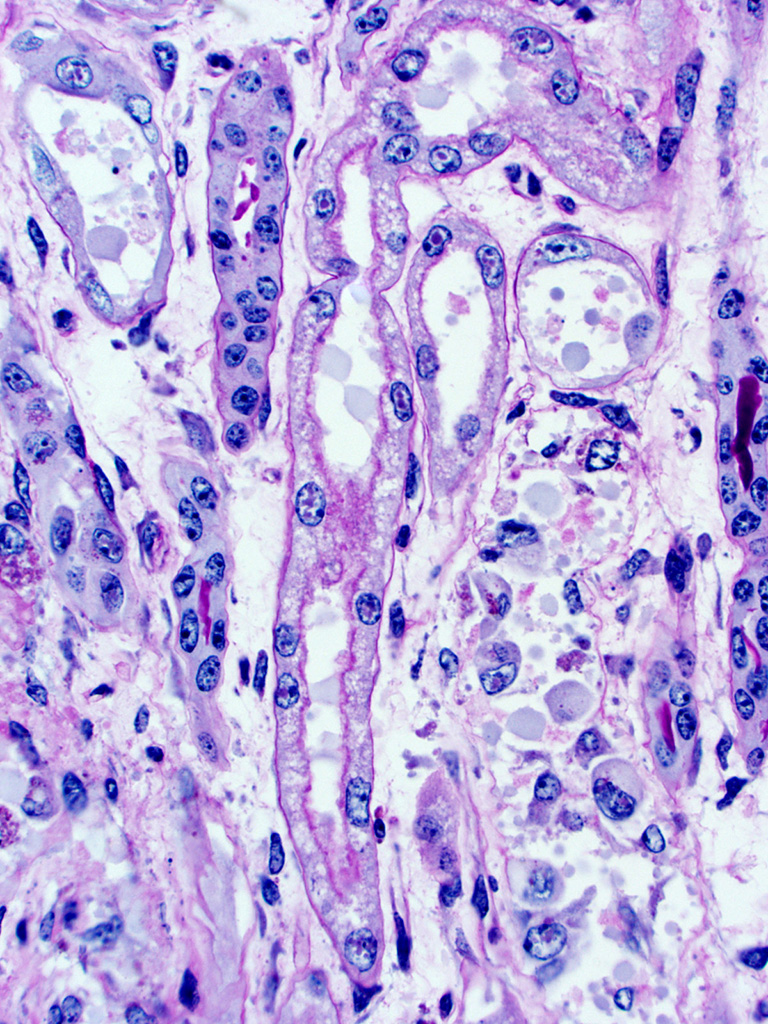
Kidney – Diffusely affecting the entire cortex, the proximal
tubular epithelium is degenerate or necrotic with marked clear cytoplasmic
vacuolization (degeneration) of the proximal convoluted tubule (PCT) segments.
The PCT have cuboidal epithelium with a discernible brush border and uniform,
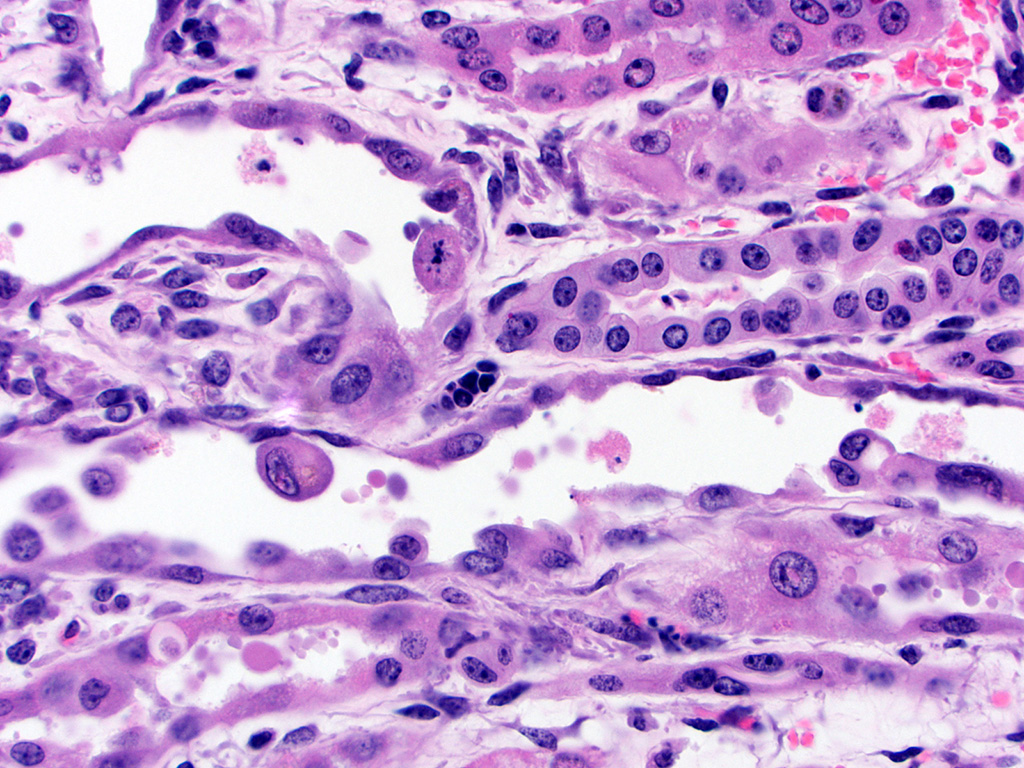
basally located nuclei frequently containing aggregates of 2-5 micron diameter,
eosinophilic globular material displacing but not marginating the chromatin.
Within the PCT lumina, there is eosinophilic material. The plump vacuolated
cells of the PCT transition to the proximal straight tubule (PST) segments,
which are lined by variably cuboidal to flattened epithelium, often with large
nuclei (up to 50 microns in diameter or approximately 3-4 times the size of a
normal renal tubular cell) and occasionally multiple nuclei, which contain
similar eosinophilic intranuclear material as well as mitoses (interpreted as
mixed degeneration and regeneration). There are areas of tubular epithelial
necrosis and loss within the PST segment which is characterized by exposure of
the basement membrane and numerous cells with pyknotic or karyorrhectic nuclei
which are sloughed into the tubular lumen, admixed with small amounts of
eosinophilic globular material. There are minimal histologic changes
discernible in the medullary components (Loops of Henle, collecting ducts). The
distal straight and convoluted tubules (DST, DCT) have uniform cuboidal
epithelium with slightly basophilic cytoplasm, central located nuclei which
rarely have eosinophilic intranuclear material but moderate anisokaryosis, no discernible brush border
and often have homogeneous pale to brightly eosinophilic material in the tubule
lumen (high protein-content fluid). Occasionally, there are small aggregates of
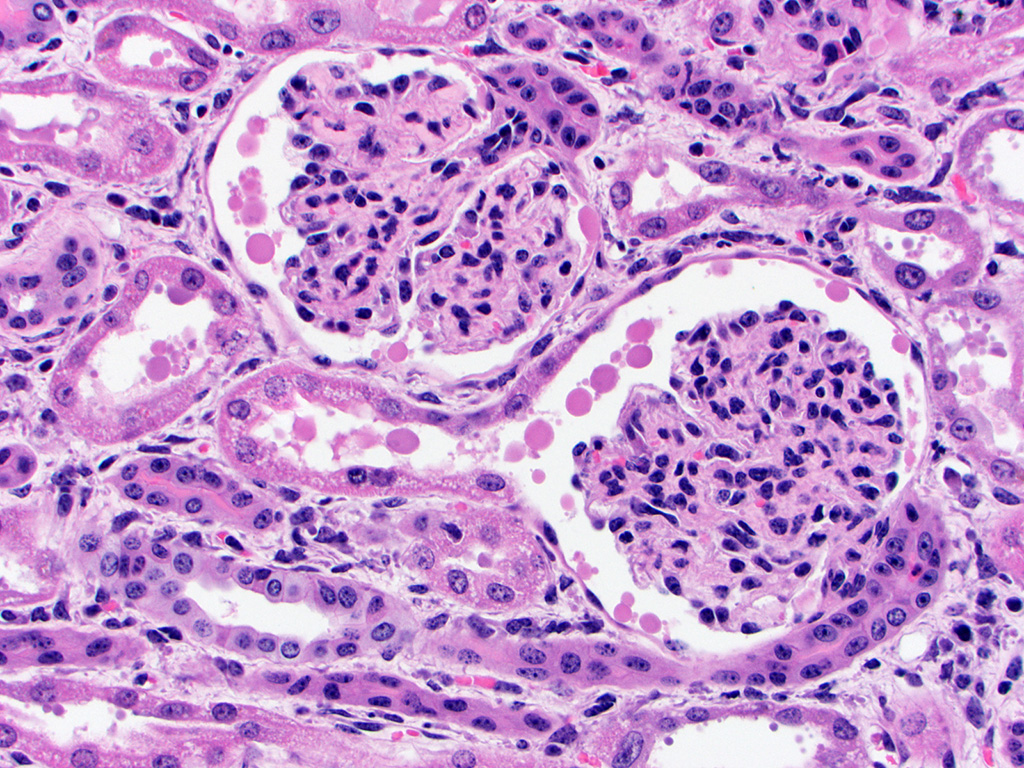
basophilic granular material within the tubular lumina (mineral). The glomeruli
are condensed and occasionally have slightly increased pale eosinophilic
material expanding the mesangium. The interstitium of the renal cortex is
expanded by clear to amphophilic, occasionally granular material (edema fluid)
and mildly increased amounts of immature collagen and fibroblasts (fibrosis).
There are multiple nodular aggregates of moderate numbers of lymphocytes,
smaller numbers of numbers of plasma cells and macrophages within the cortical
interstitium. There are multiple small areas of hemorrhage within the
perivascular space, interstitium, fibroadipose tissue subjacent to the renal
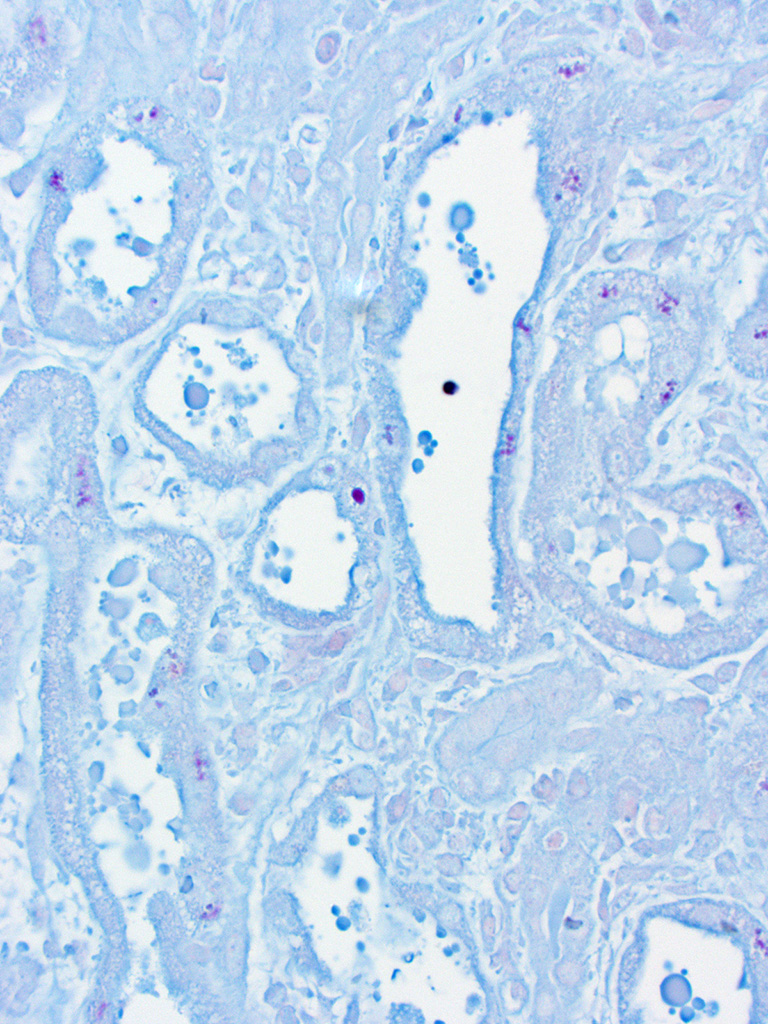
pelvis, and renal capsule. Occasionally the aggregates of eosinophilic
intra-epithelial, intra-nuclear material were strongly acid-fast positive and
frequently were moderately PAS positive.
Morphologic Diagnosis:
Kidney, tubules – tubular degeneration and necrosis, diffuse,
marked, acute, with intra-epithelial intra-nuclear eosinophilic material
(occasionally acid-fast and frequently PAS positive), tubular regeneration, and
interstitial edema and hemorrhage Kidney – nephritis, interstitial, lymphocytic, multifocal, mild,
chronic
Lab Results:
Clinical pathology:
*Reference intervals were not provided for yak; references ranges
in parentheses are for cattle tested at the referral hospital for general
comparison only
Significantly abnormal values on presentation:
BUN – 128 mg/dL (10-24)
Creatinine – 17.1 mg/dL (0.6-1.3)
Calcium – 7.6 mg/dL (8.1-10)
Phosphorus – 11.6 mg/dL (3.4-7.7)
Total protein – 4.7 mg/dL (6.2-8.9)
Albumin – 2.8 mg/dL (3.2-4)
HCT – 18% (25-47)
Values on the day of euthanasia (7 days later):
BUN – 119 mg/dL
Creatinine – 20.1 mg/dL
Calcium – 8.2 mg/dL
Phosphorus – 10.2 mg/dL
Total protein – 3.4 mg/dL
Albumin – 1.9 mg/dL
PCV – 12%
Microbiology:
Antemortem aerobic culture of trans-tracheal wash: Pseudomonas
aeruginosa
Postmortem aerobic culture of lung: Pseudomonas aeruginosa
Postmortem aerobic culture of kidney, spleen and liver: No growth
Molecular diagnostics:
Kidney was submitted for Leptospira species PCR (16s rRNA):
negative
Tissue homogenate of lung and spleen was submitted for BVD PCR:
negative
Toxicology:
Liver was submitted for toxic element screen:
Lead detected at 62 ppm (wet tissue basis) (<1 ppm considered
normal for cattle, >10 ppm liver lead is consistent with toxicosis)
Condition:
Lead toxicosis, yak
Contributor Comment:
This
case is an example of acute tubular necrosis in a milk-fed, domestic yak calf (
Bos
grunniens) due to acute-to-subacute lead toxicosis. This animal was exposed
to lead by ingestion of lead-based paint (gray paint chips in the rumen) chewed
from the siding of the adjacent building. The clinical presentation of
ruminants with lead toxicosis is often one of neurologic deficits or altered mentation. Histologically, there may be cerebral edema and laminar necrosis;
9
however, this yak did not have gross or histologic evidence of notable cerebral
edema or necrosis and, other than lethargy and an inability to suckle, was not
reported to have neurologic deficits. Peripheral neuropathy, including
dysphagia and laryngeal paralysis, has been reported to be a common clinical
sign in horses with both acute and chronic lead toxicosis
11 and may
be the underlying reason for this yak's reported loss of suckle, reflux and
aspiration pneumonia which resulted in referral. Renal
changes attributed to lead toxicosis are usually reported to be mild and
occasionally evident only as eosinophilic or poorly-staining intranuclear
inclusions which are acid-fast positive but, similar to this case, severe
nephrosis has also been described in young calves.
9 The mechanism of
renal toxicity of lead is known to involve numerous pathways. Structural
mitochondrial degeneration occurs (mitochondrial swelling and distortion of cristae)
as well as decreased activity of mitochondrial heme-pathway enzymes,
5
which interfere with cellular energy production needed to maintain homeostasis
and fuel active transport processes vital to renal tubular cell function. Lead
also impacts nuclear function through altered gene expression
5 and
is histologically and ultrastructurally evident as lead-protein inclusion
bodies in some cases. In-terestingly, not all of the histologically-evident
eosinophilic intranuclear material in this
case was acid-fast positive but the majority was variably PAS-positive, sug-gesting
that many of the inclusions are high in glycogen or other carbohydrate-rich
compounds but did not necessarily contain lead complexes. Electron microscopy
was performed in this case and solitary or multiple, variably-sized
electrodense in-clusions were present in the nuclei of some renal tubular
epithelial cells, however the inclusions incorporated less fibrillar material
than expected based on previously published reports of lead inclusions.
3,12
This may be due to the fact that this was a diagnostic case and the tissue was
formalin-fixed prior to gluteraldehyde fixation, which may have introduced
artifactual changes. Additional information regarding the composition of
inclusions could have been obtained from energy-dispersive x-ray spectroscopy
(EDS) but this capability is not available within the electron microscope at
the submitting institution. The specificity of renal acid-fast positive
inclusions for lead intoxication has been reported to be high in cattle
13
but renal intranuclear acid-fast inclusions should be further investigated.
Other general me-chanisms of lead toxicity likely impacting renal function
include lead competitively binding in place of calcium, altered calcium
regulation, and structural and functional alteration in cellular enzymes.
10
Young animals are
known to absorb ingested lead more efficiently than adults7 and
there are numerous dietary factors known to impact absorption of ingested lead.1 Many studies on
lead absorption are rodent-based; however, studies in cattle identified
significant differences in lead absorption from milk-fed vs. grain and hay-fed
calves15 and in absorption based on levels of lactose in the diet.14 Calves fed
exclusively milk and calves fed elevated lactose levels with grain absorbed
more lead than calves not receiving milk or without high levels of lactose
supplemented to grain. These diet-related factors may be a major factor for
susceptibility in young animals, and in this case the affected yak was on a
milk-only diet.
The underlying
cause and significance of the lymphocytic interstitial nephritis in this case
is unknown.
JPC Diagnosis:
1. Kidney: Tubular degeneration, necrosis, regeneration, and proteinosis,
diffuse, marked with tubular casts and intranuclear, eosinophilic inclusion
bodies within renal tubule epithelial cells.
2. Kidney: Nephritis, cortical, interstitial, chronic, multifocal, mild.
Conference Comment:
In
addition to domestic animals, wildlife may also be exposed to lead from various
sources. Ingestion of lead is the most common route of exposure, although
toxicity due to lead-containing shot is also common (but likely poses a low
risk for lead toxicity).
8 Lead
exposure is of particular concern in wild avian species where it may affect a
variety of birds ranging from waterfowl to bald eagles. In avian species, lead
can inhibit enzymes involved in hemoglobin synthesis and when exposed at high
levels, may result in anemia. Lead toxicity is most often a chronic condition
and as such, affected birds are often debilitated and in poor body condition.
6
Gross lesions may include esophageal, ventriculus and pro-ventriculus impaction
with food, gall bladder distension, pale streaks in the myocardium and muscle
of the ventriculus indicative of necrosis, as well as pallor of internal
organs. Eosinophilic lead inclusions in the nuclei of proximal tubule
epithelium also occur in the kidney of birds and, while this finding is
specific for lead poisoning, it may not be present in all cases. Although not
necessarily specific for lead toxicity, other histologic changes in affected
birds may include hepatic hemosiderosis, fi-brinoid necrosis of arterioles, en-cephalopathy
and peripheral neuropathy. The highest concentration of lead in birds is found
in the bone, liver and kidney; lead levels in bone decline much slower than
soft tissues and thus bone serves as a much longer term location of lead
deposits.
6
Lead has no biologic function
and although lead can exert its toxic effects via a variety of mechanisms, its
competition with calcium in various biologic functions is of particular
importance. This can result in various pathophysiologic effects such as
inhibition of neurotransmitter release, defects in ion pumps and channels as
well as alterations in protein kinase function.
6 Lead is considered
neurotropic, although the precise reason is not well understood; neuronal
changes are non-specific and laminar cortical necrosis may be seen in some
cases.
2
In addition to the central
nervous system, lesions also occur in bone. The ch-aracteristic lesion is
referred to as a "lead line", which is a band of sclerosis located at the metaphysis
of developing bones. It is seen as an early lesion in both children and
animals. The lesion consists of persistent mineralized cartilage trabeculae
which cannot be effectively resorbed by osteoclasts in spite of their apparent
abundance microscopically. Osteoclasts may also contain acid-fast intranuclear
inclusions.
4
We thank the contributor for
providing clinical pathology data and gross images with the submission, which
enhance the teaching / learning value of the case.
References:
1.
Barltrop D, Khoo HE. The influence of nutritional factors on lead absorption.
Postgrad Med J. 1975;51(601):795-800.
2. Cantile C, Youssef S. Nervous
system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of
Domestic Animals. 6th ed. Vol 1. St. Louis, MO: Elsevier; 2016:316-317.
3.
Choie DD, Richter GW. Lead Poisoning: Rapid Formation of Intranuclear
Inclusions. Science. 1972;177(4055):1194-1195.
4. Craig LE, Dittmer KE, Thompson KG. Bones and Joints. In: Maxie MG, ed. Jubb, Kennedy, and
Palmer's Pathology of Domestic Animals. 6th ed. Vol 1. St. Louis, MO:
Elsevier; 2016:86.
5.
Fowler BA. Mechanisms of kidney cell injury from metals. Env Health Persp.
1993;100:57-63.
6.
Golden NH, Warner SE, Coffey MJ. A review and assessment of spent lead
ammunition and its exposure and effects to scavenging birds in the United
States. Rev Environ Contam Toxicol. 2016;237:123-91.
7.
Kostial K, Kello D, Jugo S, et al. Influence of age on metal metabolism and
toxicity. Env Health Persp. 1978;25:81-86.
8.
LaDouceur EE, Kagan R, Scanlan M, Viner T. Chronically embedded lead
projectiles in wildlife: A case series investigating the potential for lead
toxicosis. J Zoo Wildl Med. 2015;46(2):438-442.
9. Maxie
MG. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 5th ed. Elsevier
Saunders; 2007.
10.
Needleman H, Needleman. Lead Poisoning. Annu Rev Med.
2004;55(1):209-222.
11. Puschner
B, Aleman M. Lead toxicosis in the horse: A review. Eq Vet Ed.
2010;22(10):526-530.
12.
Richter GW, Kress Y, Cornwall CC. Another look at lead inclusion bodies. Am
J Pathol. 1968;53(2):189-217.
13.
Thomson RG. Reliability of acid-fast inclusions in the kidneys of cattle as an
indication of lead poisoning. Can Vet J. 1972;13(4):88-9.
14.
Zmudzki J, Bratton GR, Womac CW, et al. Lactose and milk replacer influence on
lead absorption and lead toxicity in calves. Bull Environ Contam Toxicol.
1986;36(1):356-363.
15. Zmudzki J, Bratton GR, Womac C, et al. The influence of milk
diet, grain diet, and method of dosing on lead toxicity in young calves.
Toxicol Appl Pharmacol. 1984;76(3):490-497.