Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 21
April 11th, 2018
CASE IV: 16N-3473 (JPC 4101312).
Signalment: 6-month-old, female, Quarter horse (Equus caballus), equine.
History: This filly came from a farm at which two other horses reportedly had neurologic disease over the past five years. The patient was grade 4/5 ataxic. Cervical radiographs were unremarkable. She was down in the trailer and was unable to stand without assistance upon arrival to the clinic. Neuroaxonal dystrophy was suspected. The animal was eventually euthanized due to the poor prognosis. Deworming and vaccination history were unknown.
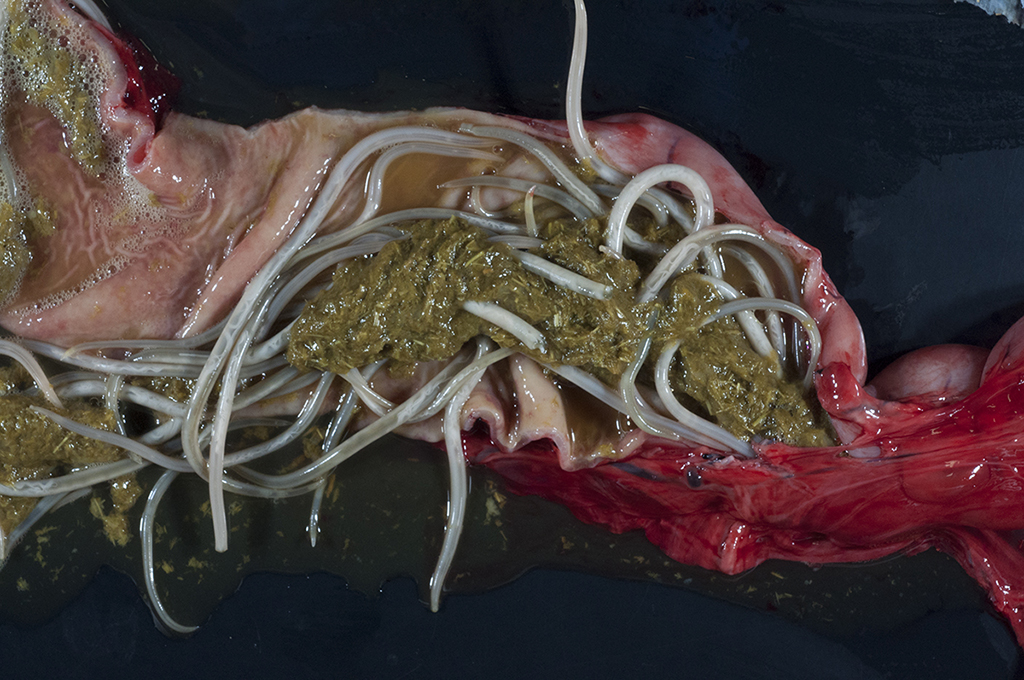
Gross Pathology: Four 0.4- to 0.5-cm in diameter ulcers were present on squamous epithelium of the cardia. Hundreds of large robust nematodes consistent with Parascaris equorum filled the duodenum and jejunum. Approximately fifty slender elongate, white nematodes consistent with strongyle larvae were present in the large intestine, most notably in the right dorsal colon. The abdominal aorta at the level of the root of the cranial mesenteric artery was segmentally thickened. Other lesions were insignificant and/or were not relevant to the presenting complaints or the slide shown here.
Laboratory Results (clinical pathology, microbiology, PCR, ELISA, etc.): None provided.
Microscopic Description:
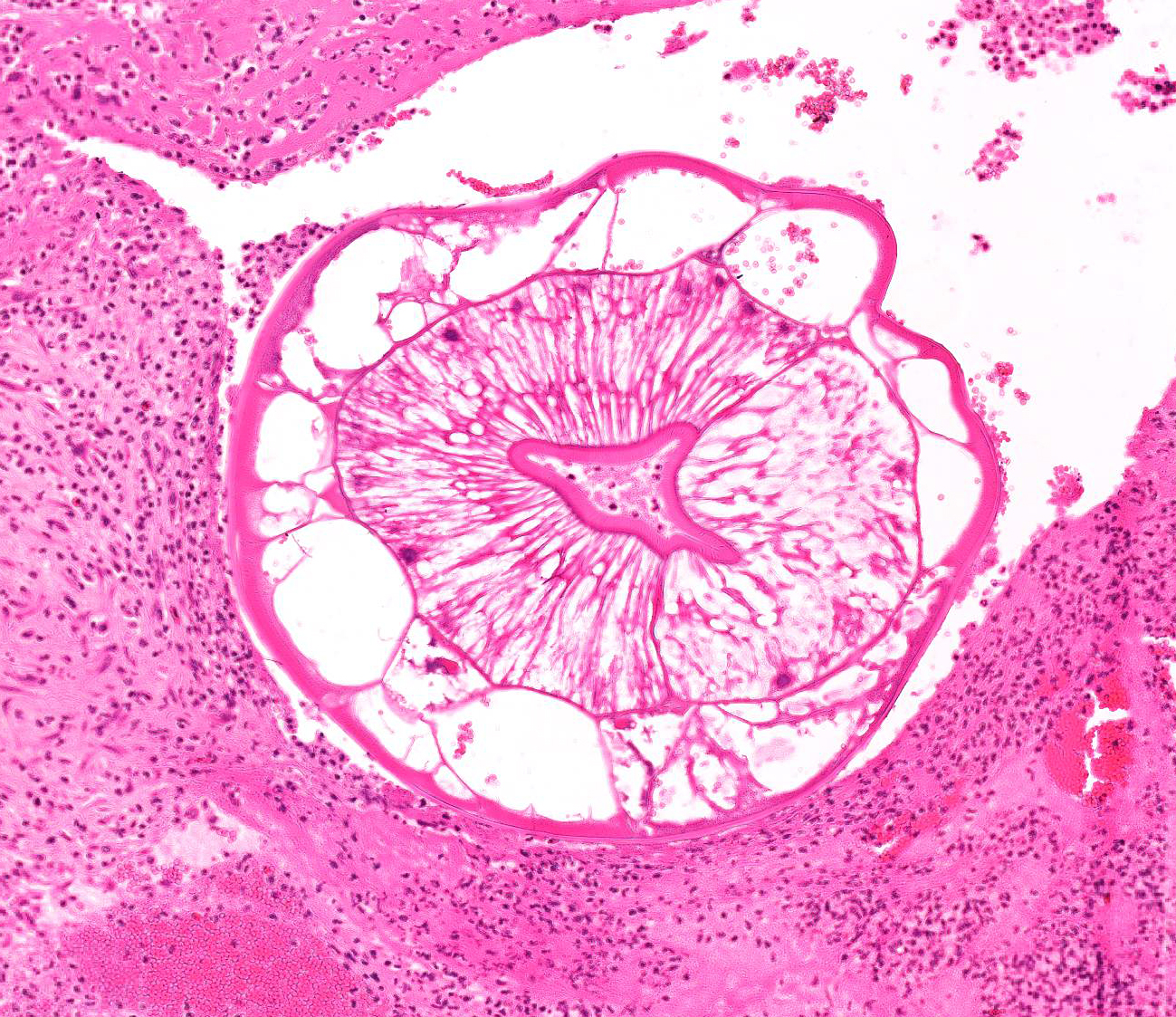
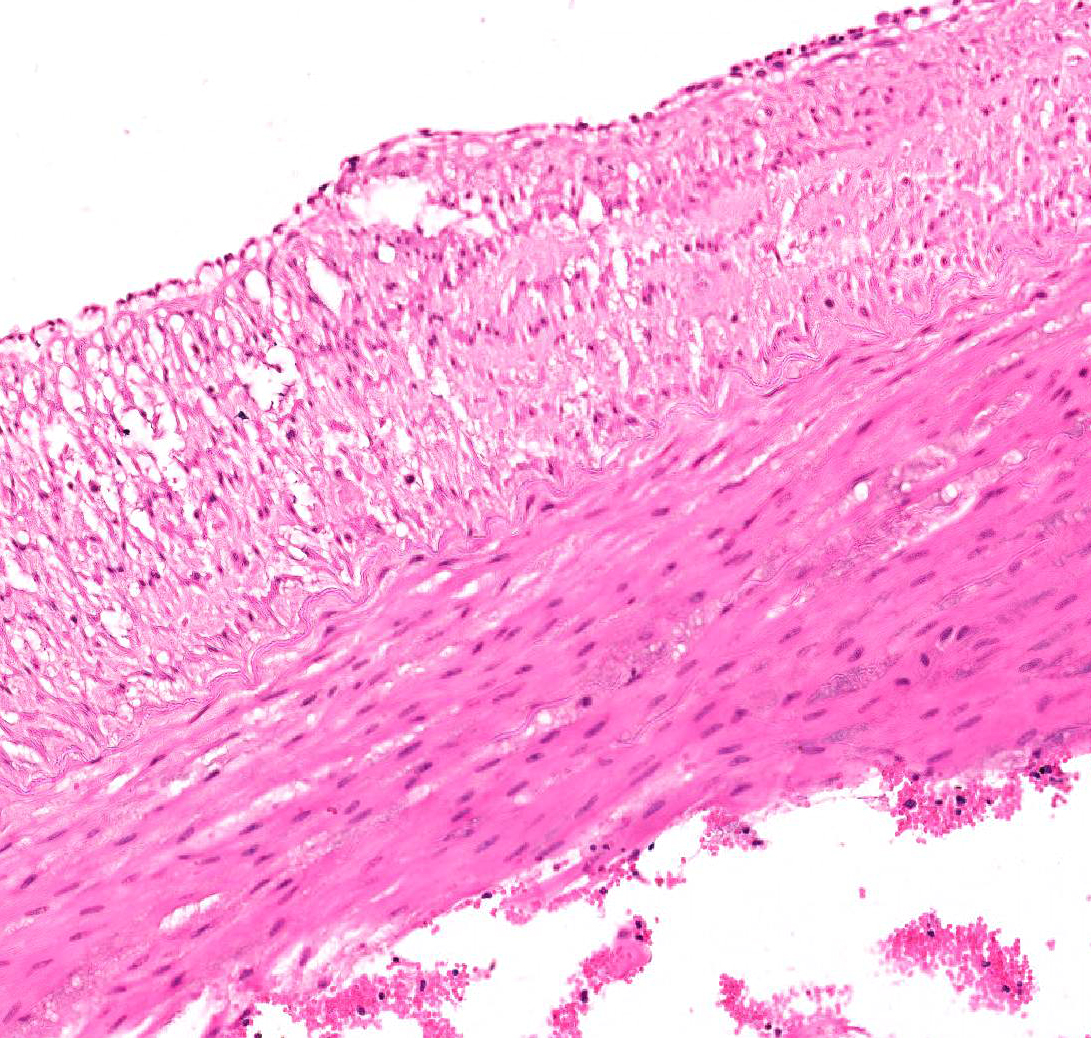
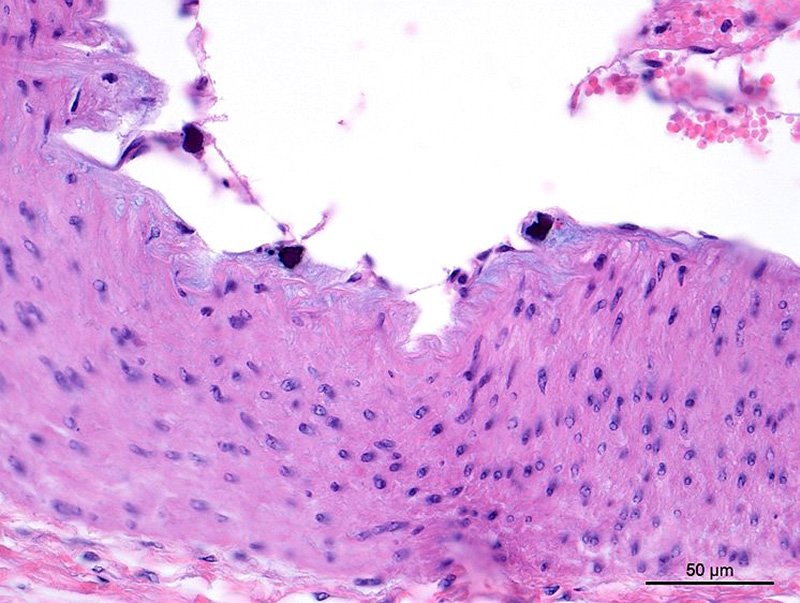
Aorta: The artery is asymmetrically thickened, and the intima is expanded and partially effaced by multifocal to coalescing necrosis, inflammation and multiple cross-sections of nematodes. The nematodes are covered by a superficial layer of fibrin with abundant erythrocytes neutrophils and small amounts of cellular debris. Endothelial cells are commonly plump and crowded along the affected surface. Endothelium is often absent in regions lined by the thrombus with adjacent nematodes. The nematodes are approximately 400 um in diameter with a smooth, glassy eosinophilic, 8.0-um-thick cuticle, platymyarian muscle, a pseudocoelom, and a large central intestine lined by a few multinucleated cells with a bright-eosinophilic brush border. The reproductive tracts are not visible or are underdeveloped (consistent with larval forms). The adjacent internal elastic lamina appears disrupted and the nearby tunica media and adventitia are multifocally vacuolated and distorted with moderate to abundant numbers of infiltrating lymphocytes, plasma cells and neutrophils. This region also contains multifocal small areas of necrotic cellular debris and some scattered erythrocytes. Similar, small aggregates leucocytes are scattered throughout the adjoining adipose tissue. The adventitia of the smaller nearby arteries are occasionally cuffed by small numbers of lymphocytes and plasma cells.
Contributor’s Morphologic Diagnosis:
Aorta (at the junction of root of cranial mesenteric artery): Severe, multifocal to coalescing, neutrophilic, lymphoplasmacytic, endarteritis with luminal thrombosis and larval nematodes (presumed Strongylus vulgaris larvae)
Contributor’s Comment: The lesions in the abdominal aorta at the junction with the cranial mesenteric artery described above are secondary to Strongylus vulgaris larvae invasion. Of the nematodes that affect horses, the small strongyle group, or cyathostomins, are found most commonly. However, S. vulgaris is the most important strongyle species affecting horses, as it causes the most damage to the host. S. vulgaris develops uniquely as it’s the only strongylid that completes a stage of larval maturation in the arterial system of the horse. L3 larvae are ingested from contaminated fields and penetrate through the mucosa to the submucosa in the ventral colon or cecum. The larvae mature and molt to the L4 stage which invade the submucosal arterioles. L4 larvae migrate in or along the intima to the cranial mesenteric artery where they mature for the next 3 to 4 months until molting into the L5 stage. L4 larvae are unable to penetrate the internal elastic lamina and therefore cannot invade the media of vessels. Blood flow to the intestinal subserosal arteries transports the L5 larvae back to the subserosa of the cecum and colon where they are encapsulated forming nodules. When nodules rupture, adult worms return to the gut lumen where they become sexually mature in another 6 to 8 weeks.6
The cranial mesenteric artery and a communicating branch, the ileo-cecocolic artery, are the vessels most commonly affected by S. vulgaris migration. Lesions associated with S. vulgaris migration have also been identified in the aorta, the celiac artery and its branches, the renal arteries, and the spermatic vessels. Lesions range from common but incidental tortuous intimal tracks to uncommon occlusive thrombosis resulting in non-strangulating intestinal infarction leading to severe colic signs. Other possible outcomes of larval migration include thickening of mesenteric vessels leading to increased pressure on the abdominal autonomic plexi, which interferes with intestinal innervation. Toxins produced from degenerating larvae can also lead to signs of colic. Aberrant migration of S. vulgaris larvae can cause CNS signs, hind limb lameness, or even renal infarction.6
Characteristic histologic findings of S. vulgaris-induced endarteritis are present in the current case and include proliferation of the intima, adventitia and endothelium; associated fibrin deposition; regions of hemorrhage and necrosis within the vessel wall, and chronic ongoing inflammation of the intima associated with the nematodes. This case also exhibits area of internal elastic lamina disruption with subsequent thickening of the media and associated inflammation. The nematode larval characteristics include a thick cuticle, platymyarian musculature, a pseudocoelom, prominent lateral cords, and an intestinal tract lined by only a few multinucleated cells with a prominent, bright-eosinophilic brush border.4
Broad-spectrum anthelmintics, primarily the macrocyclic lactones, have been used to control the effects of S. vulgaris on for approximately the last 50 years.7 Nematode control has followed interval treatment regimens involving the frequent administration of anthelmintic products based on established times for the reappearance of strongyle eggs in feces after treatment. This interval-based treatment has been successful in significantly reducing the prevalence of strongyle infections and the incidence of large strongyle-associated disease.5 Anthelmintic drug resistance, however, has been an unfortunate side-effect of this type of therapy, and there are now several reports of resistance in stronglyes after ivermectin or moxidectin administration in numerous countries.1,5,6,7
Due to recent regulatory changes in Denmark, anthelmintics can only be acquired with a veterinarian prescription, and only after parasitological diagnosis.4 Veterinarians in Denmark now have a more active role in regulating responsible anthelmintic drug use to reduce drug resistance. Regular interval-based treatment without attaining fecal egg counts is still commonly practiced in most countries, many of which are reporting anthelmintic drug resistance. Recently, there has been increased support for implementation of similar regulations in some of these countries.6
The presenting clinical signs in this horse were suggestive of neuroaxonal dystrophy (NAD), and lesions in the medulla oblongata and thoracolumbar regions found on postmortem histologic examination were consistent with NAD. Inflammation of the intestinal lamina propria as well as lymphadenopathy were secondary to infestation with Parascaris equorum, S. vulgaris, and small Strongyles in the small and large intestine.
JPC Diagnosis: Muscular artery: Arteritis, proliferative and necrotizing, transmural, chronic, severe with mural thrombosis and numerous larval strongyles, Quarter horse (Equis caballus), equine.
Conference Comment: Verminous arteritis is most often caused by larvae of the Strongylus and Ascaris genera.6
Table 1: Causes of verminous arteritis3,6
|
Nematode |
Location |
Species affected |
|
Angiostrongylus vasorum |
Pulmonary arteries |
Dogs |
|
Spirocerca lupi |
Thoracic aorta; esophagus |
Dogs |
|
Dirofilaria immitis |
Pulmonary arteries; right heart |
Dogs |
|
Strongylus vulgaris |
Cranial mesenteric and ileo-cecocolic arteries |
Horses |
|
Oncocerca armillata |
Aorta |
Cattle, water buffaloes, goats, camels |
|
Elaeophora poeli |
Aorta |
Cattle and related species |
|
Elaeophora schneideri |
Common carotid arteries |
Mule deer, black-tailed deer |
|
Elaeophora bohmi |
Arteries and veins of the metacarpus, metatarsus, more distal extremities |
Austrian horses |
|
Crassicauda magna |
Mesenteric and gastroepiploic arteries; thoracic and abdominal aorta |
Cuvier’s beaked whales (Ziphius cavirostris)3 |
Equine strongylosis is caused by members of the family Strongylidae which are common nematode parasites of the cecum and colon and often present in mixed infections. There are two subfamilies: Strongylinae (large strongyles – genera Strongylus, Triodontophorus, Oesophagodontus, and Craterostomum) and Cyathostominae (small strongyles). The large strongyles are plug feeders or blood suckers, of which Strongylus sp. undergoes extensive migration with wide-reaching effects. The small strongyles feed on intestinal contents and are mostly non-pathogenic in adults.9
Of the large strongyles, Strongylus vulgaris is the most significant. Larval forms cause endoarteritis in mesenteric vessels leading to arterial infarction of the large bowel and colic. Adult forms cause anemia and ill-thrift. The terms “endoarteritis” and “endarteritis” are often used interchangeably, however, endarteritis implies that end arteries are primarily affected.6 Verminous endoarteritis is succinctly summarized by the contributor above. The other large strongyles, S. edentatus and S. equinus, rarely cause lesions. However, subserosal hemorrhagic plaques, termed hemomelasma ilei, are often attributed to trauma by migrating S. edentatus larvae but could be caused by any migrating strongyle.9
The cyathostomins, or small strongyles, are non-pathogenic as adults, but as larvae cause a clinical syndrome called larval cyathostominosis. This syndrome is caused by simultaneous emergence of larva from the deep mucosa or submucosa of the cecum and large colon. Emergence corresponds to the climate favorability for the parasites; hypobiosis or developmental inhibition occurs during cold or very hot months. It is only in late winter, spring, and early summer that encysted larvae emerge collectively to continue their development in the intestinal lumen.9
Conference participants viewed a Masson’s trichrome and the remarkable amount of fibrosis and granulation tissue that effaces the muscular artery, as well as the intimal hyperplasia seen in the section. According to the moderator, the reviewed specimen is likely the cranial mesenteric artery because it is a muscular artery. The aorta, on the other hand, is an elastic artery. Paucity of medial elastic lamina was demonstrated in this specimen using a Movat’s pentachrome stain which highlights elastin. Additionally, the presence of intimal bodies along the endothelium was noted, a common finding in large arteries of horses.2
Contributing Institution:
UC Davis School of Veterinary Medicine
http://www.vetmed.ucdavis.edu/index.cfm
References:
- Canever RJ, et al. Lack of Cyathostomin sp. reduction after anthelmintic treatment in horses in Brazil. Veterinary Parasitology. 2013; 194: 35-39.
- de Oliveira AC, Rosenbruch M, Schulz LC. Intimal asteroid bodies in horses: light and electron microscopic observations. Vet Pathol. 1985;22(3):226-31.
- Diaz-Delgado J, Fernandez A, Xuriach A, Sierra E, et al. Verminous arteritis due to Crassicauda in Cuvier’s beaked whales (Ziphius cavirostris). Vet Pathol. 2016;53(6):1233-1240.
- Gardiner CH, Poynton SL. Strongyles. In: An Atlas of Metazoan Parasites in Animal Tissues. Washington, DC; Armed Forces Institute of Pathology. 2006: 22-24.
- Matthews JB. Anthelmintic resistance in equine nematodes. International Journal for Parasitology: Drugs and Drug Resistance. 2014; 4: 310-315.
- Robinson WF, Robinson NA. Cardiovascular system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 3. 6th ed. St. Louis, MO: Elsevier; 2016:85-87.
- Sallé G and Cabaret J. A survey on parasite management by equine veterinarians highlights the need for a regulation change. Veterinary Record Open. 2015: e000104.
- Scott I., Bishop RM, and Pomroy WE. Anthelmintic resistance in equine helminth parasites–a growing issue for horse owners and veterinarians in New Zealand? New Zealand Veterinary Journal. 2015; 63: 188-198.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 3. 6th ed. St. Louis, MO: Elsevier; 2016:216-217.