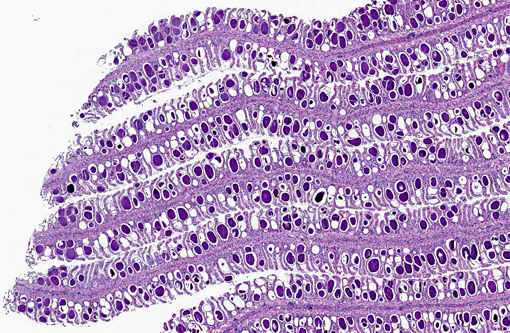
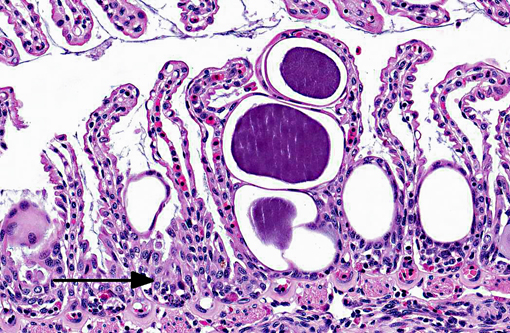
Signalment:
Female, age unknown, spotted eagle ray (
Aetobatus narinari). This 11.6 kg, 90.8 cm disc width female had been collected 5 months earlier off the Florida coast and had completed an uneventful 45-day quarantine period prior to display in a public aquarium. Acute onset of an intermittent rolling swimming pattern and flashing, typically a sign of external parasitism, was followed by progressive lethargy. Supportive treatment was initiated and a gill clip performed. Wet mount preparations of the clip revealed large numbers of large amorphous spherical bodies free and within gill lamellae. Despite treatment with oxytetracycline and chloramphenicol, the animals condition continued to deteriorate and euthanasia was elected.
Gross Description:
Gross necropsy findings were minimal. External changes were limited to mild pallor and mottling of the gills. Internally, the gastrointestinal tract was devoid of contents. The liver was unusually small, firm and tan for an elasmobranch species and failed to float in formalin.Â
Histopathologic Description:
Approximately 60-80% of lamellar troughs were occluded by one or more well delineated, 50-200 μm, amorphous, pale basophilic inclusions that markedly distended lamellar epithelial cells. Additional changes included widespread mild to occasionally moderate epithelial cell hypertrophy and hyperplasia, with patchy multifocal lamellar fusion. Multifocal infiltrates of small to moderate numbers of mixed inflammatory cells, predominated by lymphocytes and granulocytes, were located primarily in gill filaments and at the bases of lamellar toughs.
Morphologic Diagnosis:
Gill: Multifocal, mild, epithelial hypertrophy and lamellar fusion, with widespread intraepithelial amorphous basophilic inclusions and subacute, mild to moderate, filamental bronchitis.
Lab Results:
Aerobic cultures of liver, spleen and kidney were negative. PCR using pan-chlamydial primers for the 16S rRNA gene sequence produced a 276 bp product with 84% identity to
Candiditus Piscichlamydia salmons.
Condition:
Epitheliocystis
Contributor Comment:
Microscopic findings were consistent with epitheliocystis disease and supported by PCR results indicating 16s rDNA homology with other piscine chlamydia-like agents associated with the condition.Â
Epitheliocystis was first observed by Plehn in 1920 and the first connection made with a chlamydial or rickettsial-like agent by Hoffman in 1969. The disease is now known to affect the skin and gills of over 50 freshwater and marine teleosts, but is unusual in elasmobranch species. Although lesions are not uncommon in wild fish, they are rarely associated with mortalities and the condition is considered primarily a disease of aquaculture. Under intensive culture conditions mortalities can reach 100%, probably due to respiratory compromise, but are generally age dependent, with young fingerlings showing the greatest susceptibility. Losses are also influenced by stress factors typical of aquaculture, such as high stocking densities and suboptimal water quality conditions.(3,7)
Lesions are characterized by hypertrophied epithelial cells containing intracellular, spherical, membrane bound inclusions. Localization in the gill, as well as proliferative and inflammatory responses to the bacteria are highly variable. By electron microscopy two distinct developmental cycles exhibiting different histochemical and immunohistochemical staining properties have been identified in fish, but their significance is unclear. Cycle I is typical of chlamydia, involving reticulate, intermediate, and infectious elementary bodies that all possess distinct compact nucleoid regions. Microscopically, this cycle has been associated with granular inclusions in young fish during epizootics. Cycle II involves elongate forms lacking nucleoids and is reported to be more common in older animals.(3)
Growing molecular evidence indicates wider genetic biodiversity and host range among the
Chlamydiales than previously recognized. This is reflected in the recent expansion of the order to include a number of new families (
Simkaniaceae, Parachlamydiaceae, Waddliaceae) and reorganization of the family
Chlamydiaceae.(2) Concomitant with this, several specific agents have been described for the first time in association with epitheliocystis, including
Candiditus Piscichlamydia salmonis in Atlantic salmon,
Candiditus Clavochlamydia salmonicola in multiple salmonid species, and a
Neochlamydia sp. in Arctic char.(4,5,6) To date, however, no piscine chlamydial agents have been isolated in culture and Kochs postulates remain unfulfilled. Diagnosis is based largely on histopathology.Â
JPC Diagnosis:
1. Gill: Lamellar epithelial hyperplasia and hypertrophy with multifocal lamellar fusion, numerous intraepithelial intracytoplasmic bacilli, and subacute branchitis.
2. Gill: Lamellar telangiectasia with multifocal thrombosis.
Conference Comment:
The contributor provides a very good summary of epitheliocystis disease in fish. In addition to the characteristics and causes of this disease, conference participants discussed the presence and role of alarm cells in the sections examined. Alarm cells (aka alarm substance cells) in fishes in the superorder
Ostariophysi (minnows, characins, catfishes etc) and the similar club cells in non-ostariophysans (perch, walleye, saugers and darters) are specialized cells found in superficial epidermis. When damaged by predatory attack or other traumatic insult, their contents are released and serve as a chemical alarm to warn neighboring fishes of danger. The pheromone released from alarm cells is referred to as Schreckstoff which means fear substance in German. Recent studies have suggested that in addition to acting as a warning system, alarm cells may also provide protection against pathogens, parasites and UV radiation that compromise the integrity of the epidermis.(1) In the sections of gill from this spotted eagle ray there were numerous cells that appeared similar to alarm cells (large, round to oval cells with centrally placed nuclei with one to two prominent nucleoli). Due to the lack of a control, participants were unable to determine if these alarm cells were present in normal numbers, or were increased in numbers as a response to chronic gill pathology. Interestingly, the chlamydial colonies appear to be infecting the alarm cells as well as the lamellar epithelium.Â
References:
1. Chivers DP, Wisenden BD, Hindman CJ, et al. Epidermal alarm substance cells of fishes maintained by non-alarm functions: possible defense against pathogens, parasites and UVB radiation.Â
Proc. R. Soc. B. Accessed online on 26 January 2013
http://people.oregonstate.edu/~blaustea/pdfs/PRSB%20-%20Chivers%20et%20al.pdf
2. Corsaro D,Greub G. Pathogenic potential of novel chlamydiae and diagnostic approaches to infections due to these obligate intracellular bacteria.Â
Clinical Microbiology Reviews. 2006;283-297.
3. Crespo S, Zarza C, Padros F, Marin de Mateo
M. Epitheliocystis agents in sea bream Sparus aurata: morphological evidence for two distinct chlamydia-like developmental cycles.Â
Diseases of Aquatic Organisms. 1999;37:61-72.
4. Draghi A, Popov VL, Kahl MM, Stanton JB, Brown CC, Tsongalis GJ, et al. Characterization of
Candidatus Piscichlamydia salmonis (Order
Chlamydiales), a chlamydia-like bacterium associated with epitheliocystis in farmed Atlantic Salmon (
Salmo salar).Â
Journal of Clinical Microbiology. 2004;42:5286-5297.
5. Draghi A, Bebak J, Popov VL, Noble AC, Geary SJ, West AB, et al. Characterization of a Neochlamydia-like bacterium associated with epitheliocystis in cultured Arctic char
Salvelinus alpinus. Diseases of Aquatic Organisms. 2007;76:27-38.
6. Karlsen M, Nylund A, Watanabe K, Helvik JV, Nylund S, Plarre H. Characterization of
Candidatus Clavochlamydia salmonicola: an intracellular bacterium affecting salmonid fish.Â
Environmental Microbiology. 2008;10:208-218.
7. Nowak BF, LaPatra SE. Epitheliocystis in fish.Â
Journal of Fish Diseases. 2006;29:573-588.