Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 6
October 3, 2018
CASE III: P3213-13 (JPC 4048997-00).
Signalment: 1½ year-old, male neutered, Domestic shorthair, (Felis catus), cat
History: This cat was reported to be normal at birth. At 7 months of age, it started showing progressive signs of cerebellar disturbance, i.e. abnormal gait and balance. Another cat from the same litter developed similar symptoms. Two MRI examinations of the head/brain were performed. The first one, on April 2013, only showed a slightly smaller than normal cerebellar size with a small suspicion of cerebellar abiotrophy. The second one, on December 2013, revealed a reduced cerebellar size with moderate diffuse widening of the cerebellar sulci, consistent with a diagnosis of cerebellar abiotrophy. The neurological examination also supported a disease process affecting all portions of the cerebellum. Due to progression of the illness, the cat was euthanized and a full necropsy was performed.
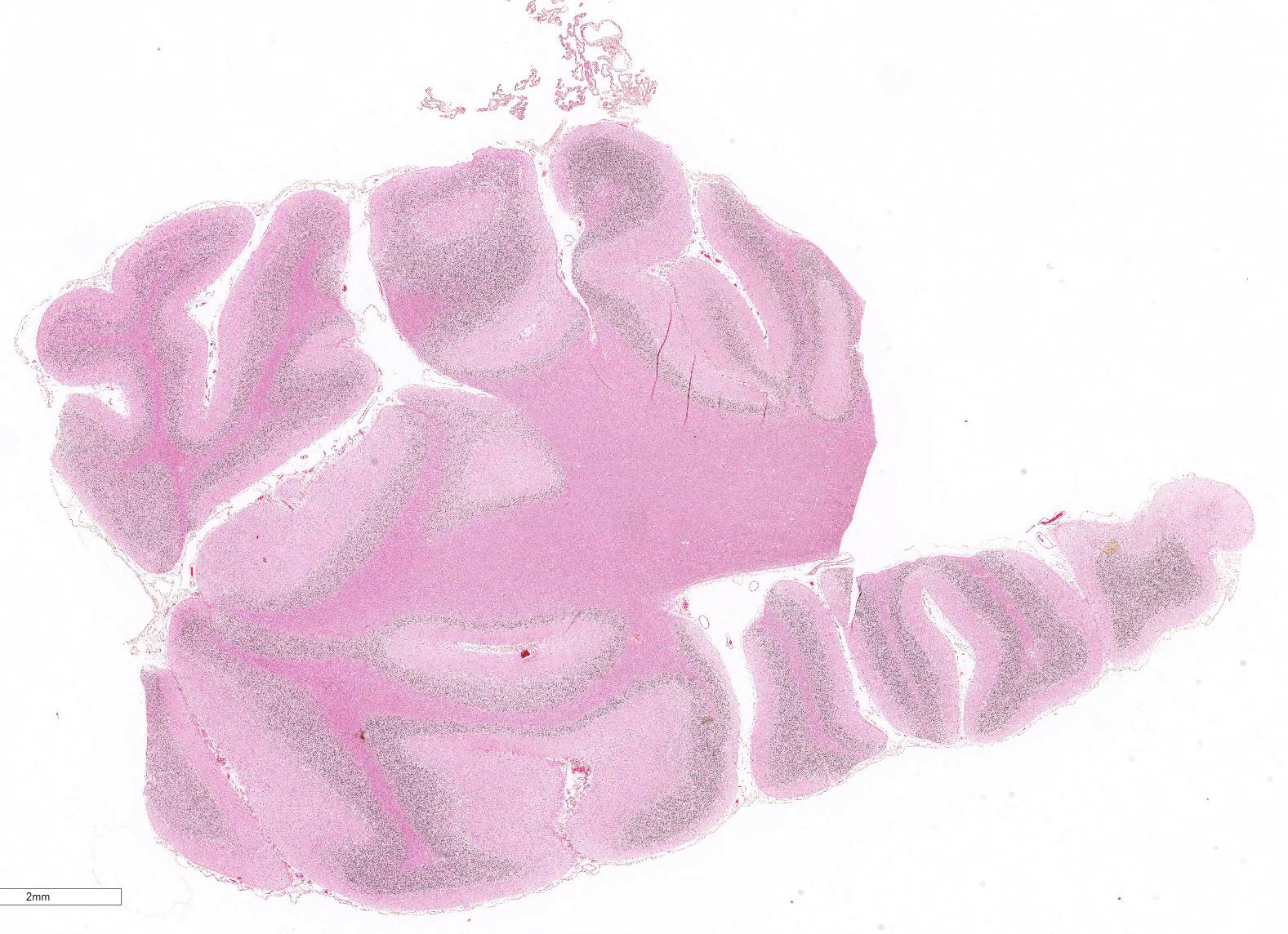
Gross Pathology: The only significant change was observed in the cerebellum which had diffusely widened sulci (more conspicuous when compared with an age-matched control), consistent with atrophy of folia.
Laboratory results: None given.
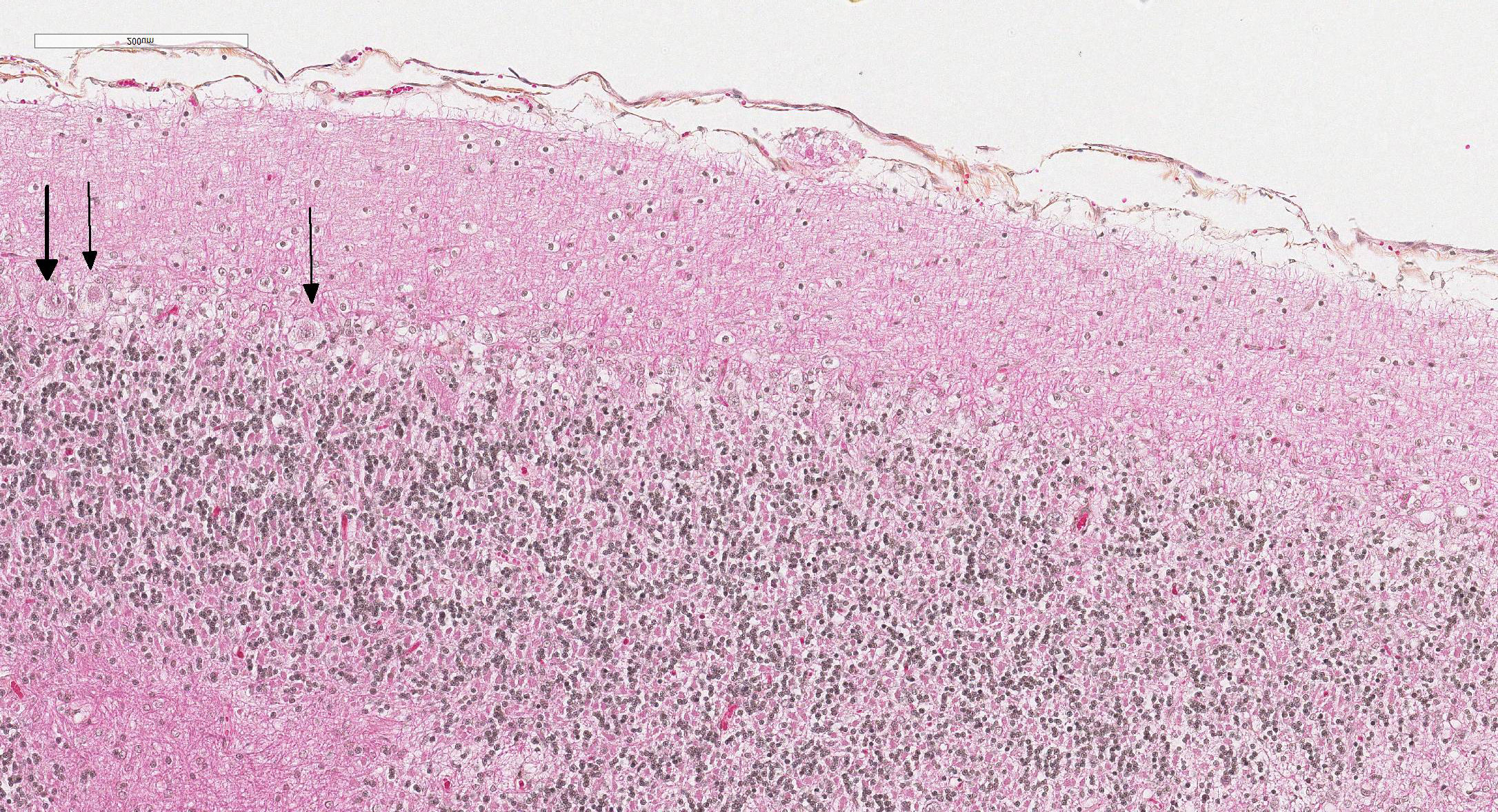
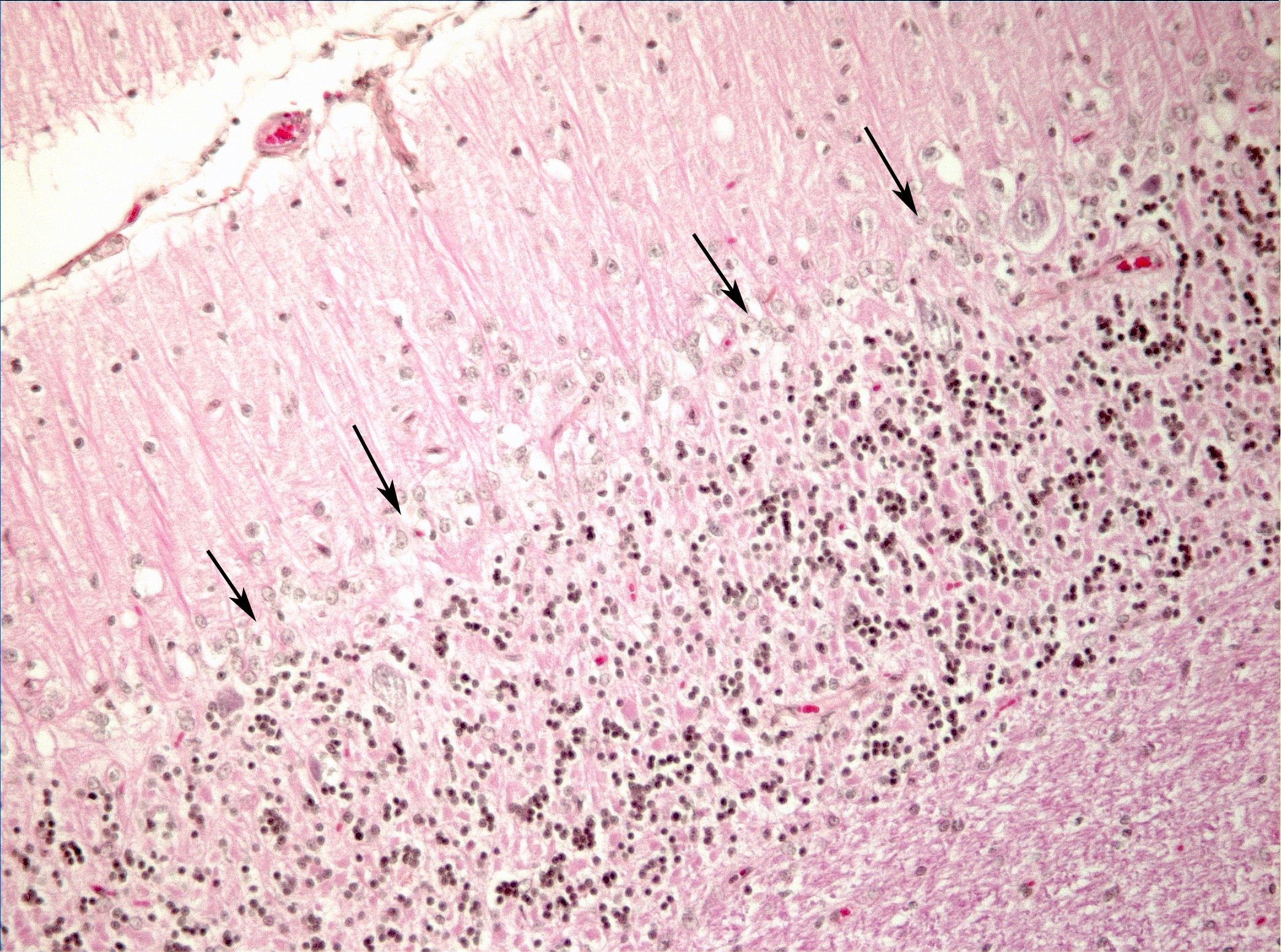
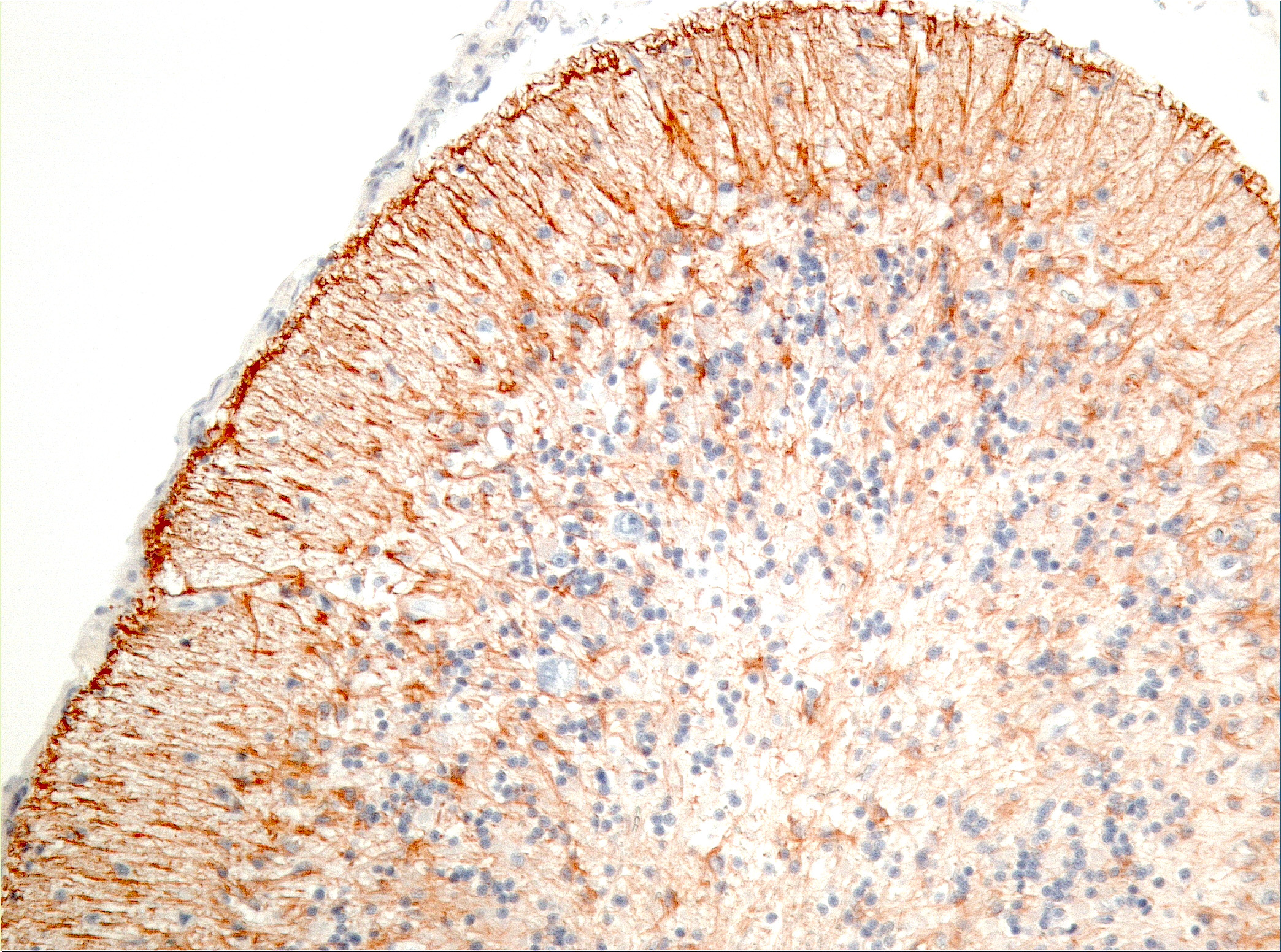
Microscopic Description: Diffusely in the cerebellar cortex, there is a severe loss of Purkinje cells, leaving multiple “empty baskets”; most of the persistent Purkinje cells have a pale and vacuolar cytoplasm (degeneration). There is an associated proliferation of Bergmann astrocytes (astrogliosis), often with prominent processes extending radially in the molecular layer to the pia mater (isomorphic gliosis); this was confirmed by GFAP immunohistochemistry. The molecular and granular layers are irregularly thin; the granular layer has a variably decreased density (loss of granule cells). Rare torpedoes can sometimes be seen. In some sections, there is Wallerian degeneration (mostly digestion chambers) in the cerebellar white matter; this change was also present bilaterally and symmetrically in the lateral and superficial portions of the medulla (not submitted). In the cerebellar nuclei (present in some sections), there is mild diffuse gliosis with some neuropil vacuolation, but no obvious neuronal changes. These lesions were present in all portions of the cerebellum.
Spinal cord (not submitted): There is bilateral and symmetrical Wallerian degeneration affecting all portions (cervical, thoracic and lumbar) of the spinal cord, with variation in the affected tracts/fasciculi depending on the level examined. In some dorsal spinal ganglia, a few neurons were chromatolytic.
Contributor’s Morphologic Diagnoses: Diffuse, severe loss of Purkinje cells (cerebellar abiotrophy)
Bilateral and symmetrical degenerative myeloencephalopathy (spinal cord and medulla; not submitted)
Contributor’s Comment: Based on the lesions in the cerebellum, medulla and spinal cord, this case was diagnosed as cerebellar abiotrophy and degenerative myeloencephalopathy, consistent with spinocerebellar ataxia; a genetic component is strongly suspected as one littermate had similar clinical signs. The cerebellar lesion is characteristic of cerebellar abiotrophy or cerebellar cortical abiotrophy. In veterinary medicine, cerebellar abiotrophy is a rare condition described in most domestic animals, non-human primates and a few rodent species.1-12 Contrary to cerebellar hypoplasia – a condition targeting the cerebellar outer germinal layer and resulting in failure to reach normal organ size (e.g. in utero infection with feline panleukopenia parvovirus or BVDV) – cerebellar abiotrophy is characterized by the loss of neuronal populations after full development of the organ. Thus, a hallmark of this condition is normal function at birth, followed by progressive signs of cerebellar disease of variable onset.10
Cerebellar abiotrophy is well recognized in dogs, with reports in several different breeds (e.g. English bulldog, American Staffordshire terrier, Border collie),2,3,5,7,10 and often a suspicion of primary genetic origin. Affected animals usually show progressive neurological signs including cerebellar ataxia, head tremors and symmetrical hypermetria. At necropsy, the cerebellum is usually of normal size but shrinkage of cereberellar folia and broadening of sulci may be present in chronically affected dogs. Microscopic examination reveals an ongoing loss of neurons associated with reactive gliosis. Purkinje cells are severely depleted, leaving clear space (“empty baskets”); Bergmann astrogliosis is a common associated finding. Depletion of the different neuronal populations eventually leads to shrinkage of the molecular and granular layers.10
In cats, cerebellar abiotrophy is uncommon, with only a few cases published1,6,9,10,11,12. However, the present feline case is to our knowledge unique as reported feline cases did not describe spinal cord lesions.
Contributing Institution:
Université de Montréal, Faculty of veterinary medicine, St-Hyacinthe, Quebec, Canada. http://www.medvet.umontreal.ca
JPC Diagnosis: Cerebellum: Purkinje cell degeneration and loss, diffuse, severe, with marked granular cell loss and Bergmann gliosis.
PC Comment: Cerebellar cortical degeneration, also referred to as cerebellar abiotrophy, encompasses a group of diseases characterized by degeneration of formed neurons within the cerebellar cortex. As opposed to cerebellar hypoplasia, in which viral infection in utero damages the external granular cell layer (the source of formation of Purkinje cells and neurons that popular the granule cell layer), the cerebellar cortex is grossly and histologically normal at birth, and progressively degenerates over time. While many of the mutations associated with both autosomal dominant and recessive forms of cerebellar cortical degeneration (CCD) have been elucidated in humans, this work is yet in its early stages in veterinary medicine. Mutations in the SEL1L gene have been associated with early onset progressive cerebellar ataxia in Finnish Hounds, and PARK2 gene mutations are common to hereditary striatonigral and cerebello-olivary degeneration in Kerry Blue Terriers and humans.
In the absence of such genetic information, most forms of CCD in veterinary medicine continue to be classified largely based on the locations in which degenerative changes are noted. Most cases of cerebellar cortical degeneration involve Purkinje cells, and the spontaneous depletion of granule cells (neurons populating the adjacent granular cell layer) is often a concurrent, although less obvious finding. Primary degeneration of neurons of the granule cell layer (also referred to as cerebellar granuloprival degeneration) has been reported in a number of canine breeds, including the Coton de Tulear (see WSC 2016-2017, Conference 12, Case 1). A number of syndromes, such as hereditary striatonigral and cerebello-olivary degeneration, as mentioned above, involve neuronal degeneration outside of the cerebellar cortex as well.
While well-documented in a wide variety of dog breeds, far fewer cases of cerebellar abiotrophy have been identified in cats. The disease has been reported both in kittens and in adult cats, with adult-onset “late” onset disease appearing in animals from 1.5 to 9 years. (Biolatti) A syndrome of olivopontocerebellar atrophy in two possibly related cats involved loss of neurons in the cerebellar cortex, olivary complex, and the pontine nuclei in an unusual orderly and progressive fashion. Subtle differences between the human and the feline disease exist, to include depletion of the granular cell layer in the feline variant and an absence of ubiquitinated intranuclear inclusions within degenerating Purkinje cells. A report of three sibling kittens described severe Purkinje cell loss as well as vacuolation of the neuropil in the cervical spinal cord (as described by the contributor in this case.)
Prominent in this section is the proliferation of Bergmann’s glia (often referred to as Bergmann’s astrocytes) in the areas of Purkinje cell loss. The cell bodies of Bergmann’s glia are found in the Purkinje cell layer, and their processes extend through the molecular layer with endfeet located on the pia mater. In additional to traditional tasks of gray matter protoplasmic astrocytes following injury to Purkinje cells, they have numerous other functions. In the developing cerebellum, they contribute to the layering of the cerebellar cortex - proneurons migrate along their processes from the external germinal layer to populate the granule cell layer. At the late stage of Purkinje cell development, Bergmann’s glia form an intricate interwoven network to provide physical and trophic support to the developing Purkinje dendrite tree. Bergmann’s glia even assist in information processing in the mature molecular layer – clearing neurotransmitters from synaptic cleft (particularly excitotoxic glutamates), buffering potassium, and even coupling neuronal activity with energy supply and blood flow.
The moderator noted the marked depletion of granular cells as well as that of Purkinje cells, but identified the Purkinje cell loss as the primary lesion, both in terms of importance to the clinical picture as well as the first of the two lesions to occur temporally.
References:
- Biolatti C, Gianella P, Capucchio MT, Borrelli A, D’Angelo A. Late onset and rapid progression of cerebellar abiotrophy in a domestic shorthair cat. J Small Anim Pract. 2010;51:123-126
- Buffo A, Rossi, F. Origin, lineage, and function of cerebellar glia. Prog Neurobiol, 2013; 109:42-63.
- Gandini G, Botteron C, Brini E, Fatzed R, Diana J, Jaggy A. Cerebellar cortical degeneration in three English bulldogs: clinical and neuropathological findings. J Small Anim Pract. 2005;46:291-294
- Gumber S, Cho DY, Morgan TW. Late onset of cerebellar abiotrophy in a Boxer dog. Vet Med Inter. 2010; Article ID 406275
- Mouser P, Lévy M, Sojka J.E, Ramos-vara J.A. Cerebellar abiotrophy in an Alpaca (lama pacos). Vet Pathol. 2009,46:1133-1137
- Olby N, Blot S, Thibaud JL, et al. Cerebellar cortical degeneration in adult American Staffordshire terriers. J Vet Intern Med. 2004;18:201-208
- Résibois A, Poncelet L. Olivopontocerebellar atrophy in two adults cats, sporadic cases or new genetic entity. Vet Pathol 2004,41:20-29
- Sandy JR, Slocombe RF, Mitten RW, Jedwab D. Cerebellar abiotrophy in a family of border collie dogs. Vet Pathol. 2002;39:736-738
- Sato J, Sasaki S, Yamada N, Tsuchitani M. Hereditary cerebellar degenerative disease (cerebellar cortical abiotrophy) in Rabbits. Vet Pathol. 2012,49:621-628
- Shamir M, Perl S, Sharon L. Late onset of cerebellar abiotrophy in a Siamese cat. J Small Anim Pract. 1999;40:343-345
- Summers BA, Cummings JF, DeLahunta A. Degenerative diseases of the central nervous system, neuronal abiotrophy. In: Summers BA, Cummings JF, DeLahunta A . ed. Veterinary Neuropathology. St Louis, MO: Mosby-Year Book; 1995:300-347
- Taniyama H, Takayanagi S, Izumisawa Y, Kotani T, Kaji Y, Okada H, Matsukawa K. Cerebellar cortical atrophy in a kitten. Vet Pathol. 1994;31:710-713
- Willoughby K, Kelly D.F. Hereditary cerebellar degeneration in three full sibling kittens. Vet Rec. 2002,151:295-298