CASE 3: A19-7646 (4134509-00)
Signalment:
7 months, intersex, English Bulldog, Canis familiaris, canine
History:
A phenotypically female dog was presented for routine ovariohysterectomy. The surgeon/submitting veterinarian reported 'abnormal uterine structure' and submitted the spay specimen in formalin.
Gross Pathology:
The uterus was small for the age of the dog. Both uterine horns had segmental flattening or narrowing.
Laboratory results:
N/A
Microscopic description:
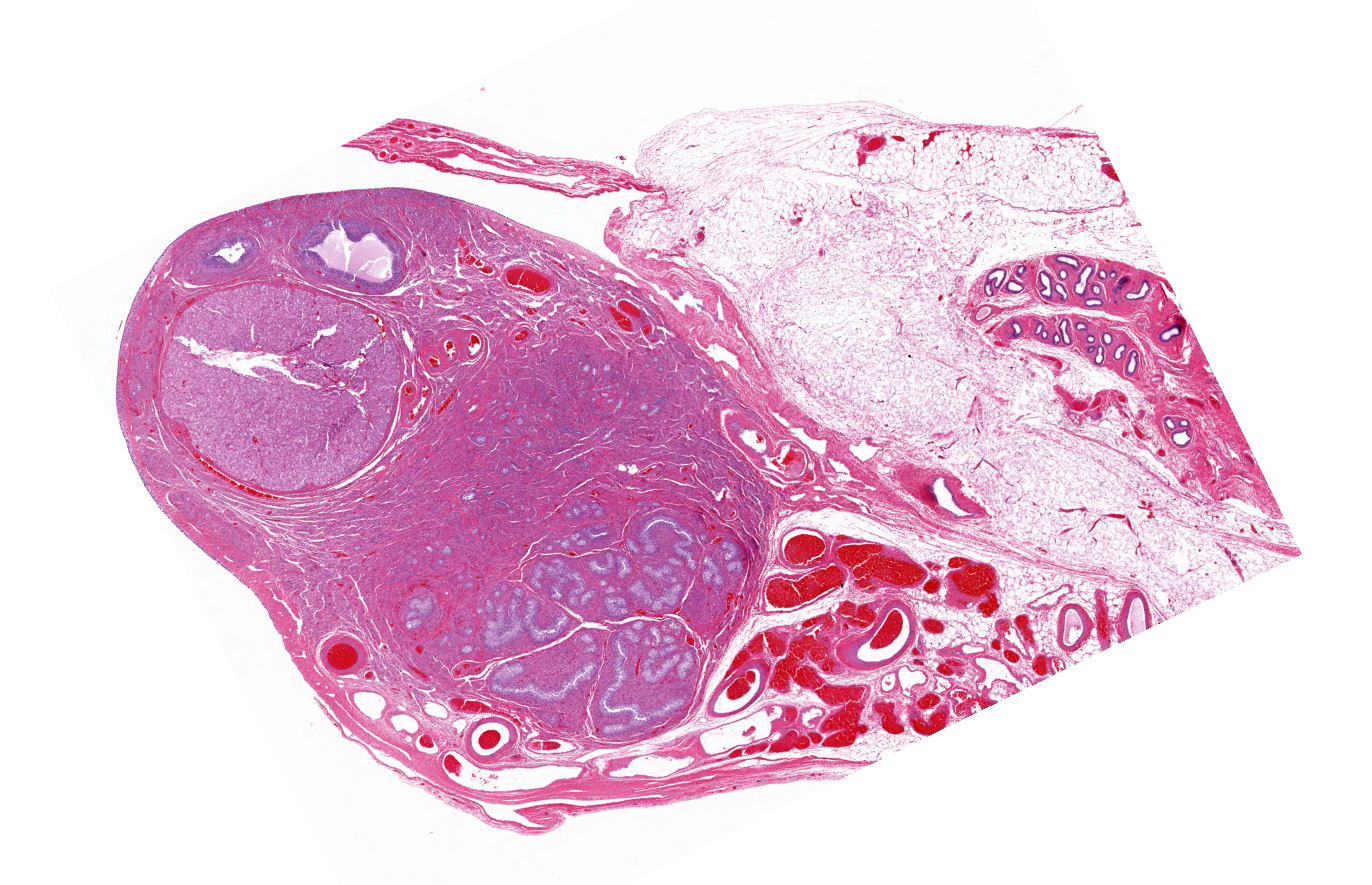
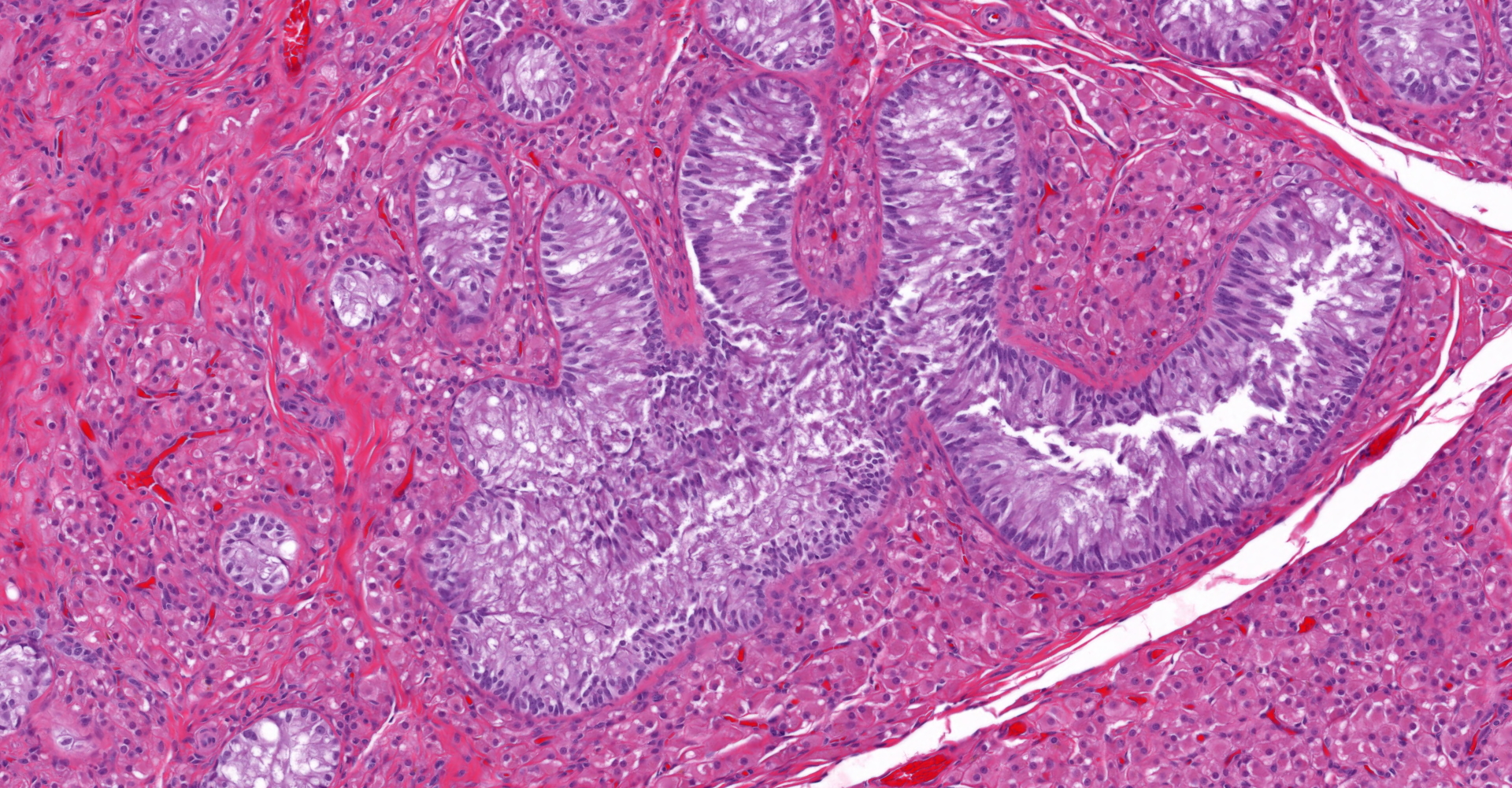
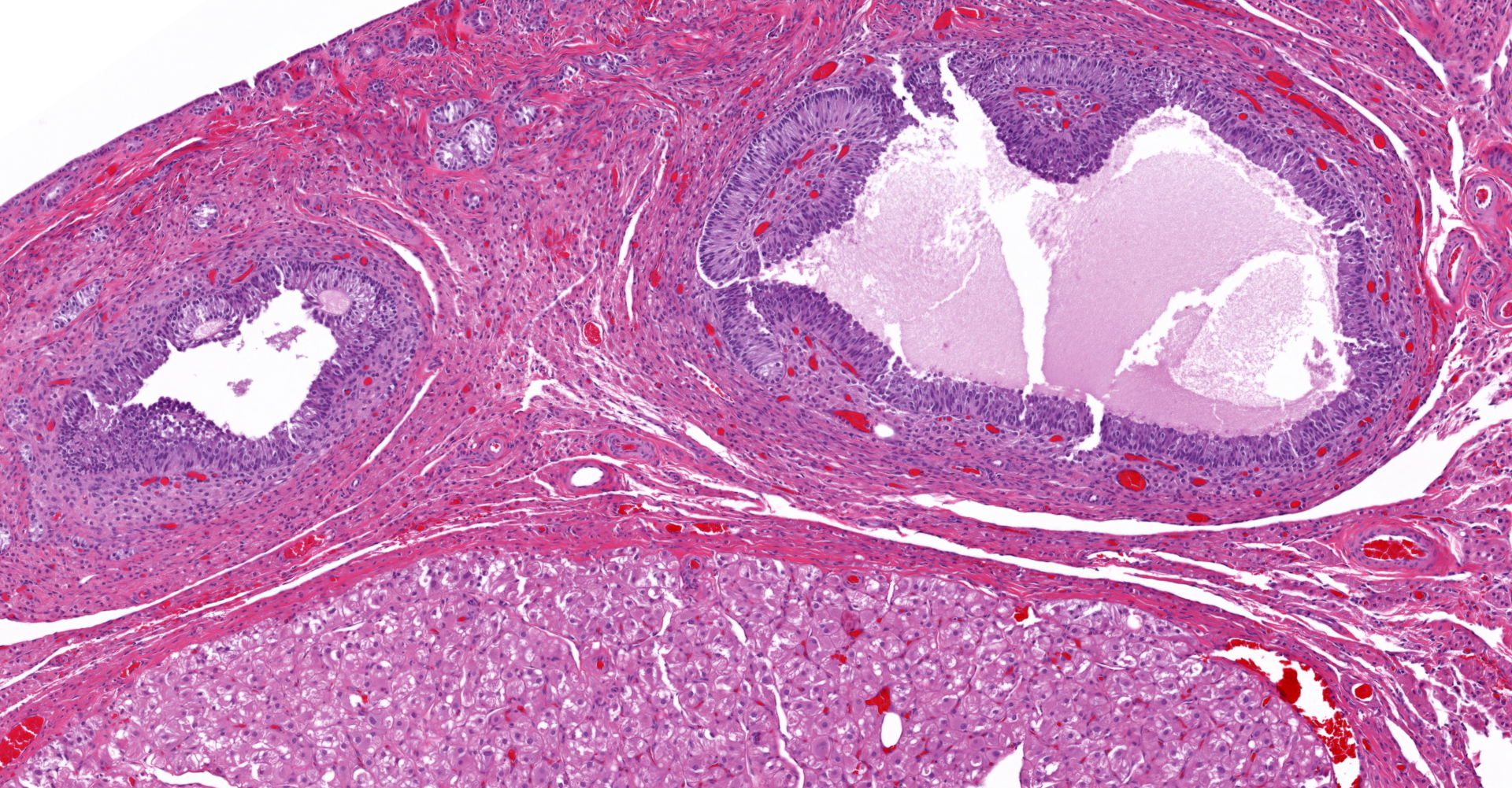
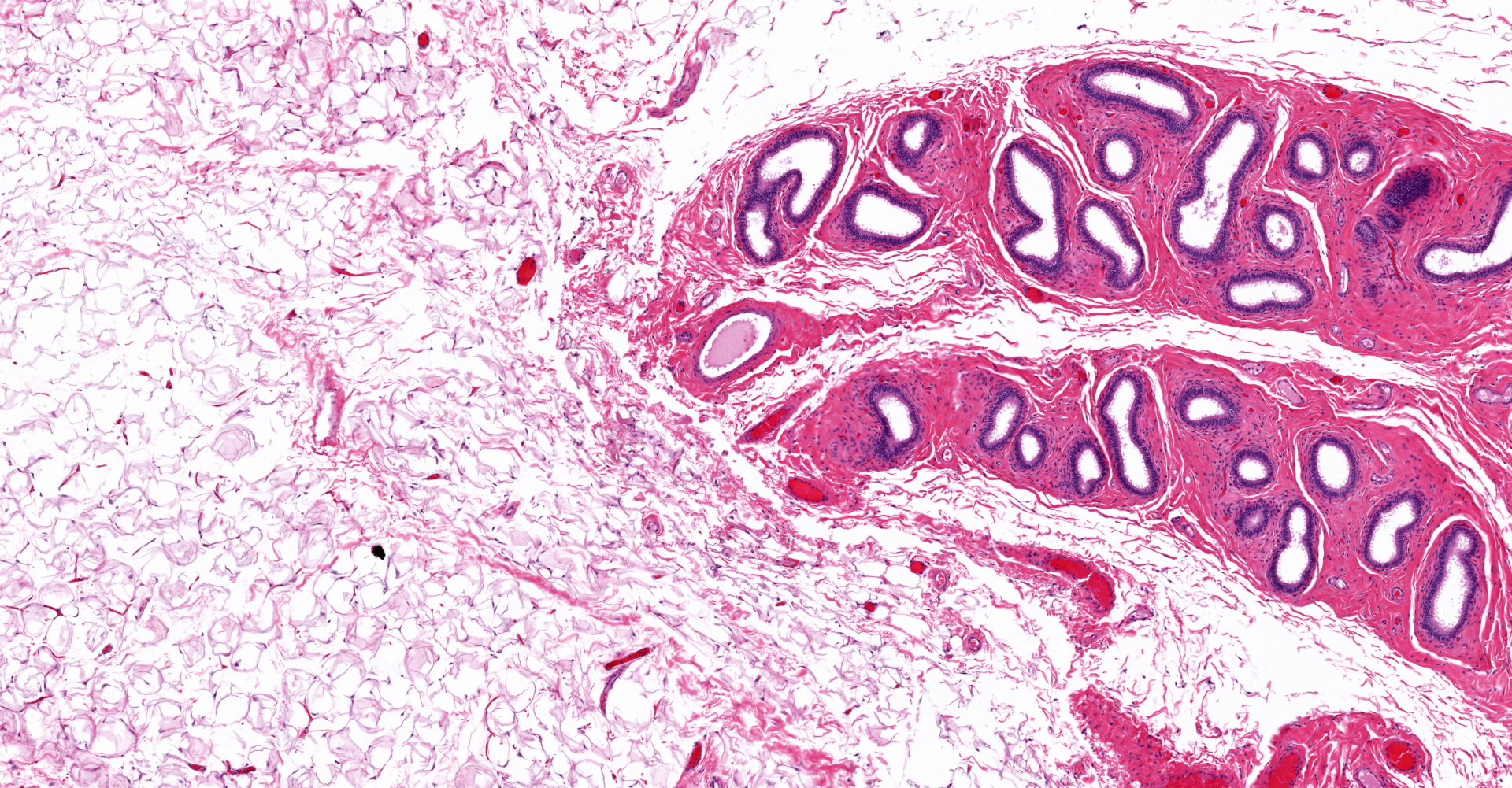
Both gonads were ovotestes. The ovarian component, toward one end of the section, included follicles in various stages of development and two small corpora lutea. The testicular component consisted mainly of interstitial cells with scattered seminiferous tubules lined exclusively by Sertoli cells. One gonadal section includes pampiniform plexus and epididymis. The uterus (not included in the submitted slide) was poorly developed with few endometrial glands and small diameter. Some segments of both uterine horns lacked an apparent lumen, depending on the plane of section.
Contributor's morphologic diagnosis:
Ovotestis
Contributor's comment:
This dog has ovotesticular disorder of sexual development (DSD). Both gonads were ovotestes. The uterus was hypoplastic. External genitalia were reportedly female. The sex chromosome type was not determined. Estrous cycle history was not provided, but the presence of corpora lutea indicates that ovulation had occurred. In contrast, spermatogenesis was not evident in the testicular component of the gonad.
Ovotestes have been reported in both XX and XY dogs, and in XX/XY feline chimeras.7 They can be unilateral or bilateral, but the genetic basis for their development has not been determined in dogs.3,4,7,11 Testicular or ovotesticular DSD has been identified in XX dogs of numerous breeds,7 but apparently has not been reported in the English Bulldog. In reported cases, the diagnosis was made between 6 months and 4 years age.1,2,5,6,9
Ovarian tissue is usually in the cortex of the gonad at one end, with testicular tissue in the medulla at the opposite end.3,4 In a study of gonadectomy specimens from ten phenotypic bitches with testicular or ovotesticular DSD, both gonads of 6 dogs were testes, 2 dogs had bilateral ovotestes, 1 had a testis and an ovotestis, and 1 had gonadoblastoma in one of two testes.2 In that and other studies, the testicular component of the ovotestis was unremarkable except for the lack of spermatogonia or any evidence of spermatogenesis. In contrast, the ovarian component is typically functional with follicles in various stages of development and corpora lutea.
Contributing Institution:
Purdue University
Animal Disease Diagnostic Laboratory: http://www.addl.purdue.edu/
Department of Comparative Pathobiology: https://vet.purdue.edu/cpb/
JPC diagnosis:
1. Gonad: Ovotesticular disorder of sexual development.
2. Gonad: Ovarian hypoplasia.
JPC comment:
This uncommon disorder of sexual development is not limited to canine species. While most cases of ovotestes are usually limited to having germ cells of either of one sex, there are rare reports of having male and female germ cells present. In a described case of ovotestis in a Loxechinus albus sea urchin, both spermatozoa and primary oocytes were present in close proximity to each other.8 This condition appears to be rare in Echinodermata, but ovotestis is ubiquitous and normal in Mollusca. Pulmonates (snails and slugs) are hermaphrodites, using their ovotestis as the source of both oocytes and sperm. In the case of mating, body size is usually the determining factor for which snail supplies the ova or sperm, with larger snails acting as female and smaller snails acting as male.10
Endocrine disrupting chemicals (EDC) can have an extensive effect within populations of aquatic animals. These chemicals are part of a vast array of pharmaceuticals, plastics, household products, flame retardants, and accumulate within the aquatic environment through urban and agricultural run-off. One of the more potent EDCs is 17-a-ethinylestradiol (EE2), a synthetic analog of estrogen 17b-estradiol (E2). Observed effects in fish include decreased fertility, modulation of steroid levels, feminization, and cases of intersex presentation or sex reversal. Proteomic investigation using the mangrove rivulus (Kryptolebias marmoratus), one of only two hermaphroditic vertebrates capable of self-fertilization (sister species K. hermaphroditus), showed that early exposure to EDCs significantly changed the protein profile of the brain, liver, and ovotestes. Lower doses primarily affected brain and liver proteomes, but higher doses altered the ovotestis proteome with a dose-dependent response pattern. It is likely that estrogen-dependent pathways, such as lipid-metabolism, inflammation, and the innate immune system remain affected months after exposure to EDCs.13
Recent work with rabbits has yielded a potential animal model for DSD due to mutations in the SRY gene on the Y chromosome. Inducing clustered regularly interspaced short palindromic repeats (CRISPR)/Caspase 9-mediated mutation of the HMG region of SRY, rabbits developed ovotestis, testis, ovary, and uteri simultaneously. By demonstrating the ability to manipulate these genes, this may represent an avenue for further research of disorders of sexual development in animals and humans.12
The moderator emphasized the changes in terminology for classification of these disorders. True and pseudohermaphrodite and intersex are no longer preferred terms and are replaced by a collection of characteristics and traits. Disorders of sexual development are characterized by sex chromosome, SRY status, gonad type, tubular genitalia, and external genital phenotype. Unfortunately, karyotyping was not available in this case.
References:
- Diel de Amorim M, Lerer A, Durzi T, Foster FA, Gartley CJ. Identification of ectopic ovotestis in a dog with XX ovotesticular, SRY-negative, disorder of sexual development. Reprod Domest Anim. 2018;53(3):822-825.
- Dzimira S, Nizanski W, Ochota M, Madej JA. Histopathological pattern of gonads in cases of sex abnormalities in dogs: an attempt of morphological evaluation involving potential for neoplasia. Pathol Res Pract. 2015;211:772-775.
- Foster RA. Female reproductive system and mammae. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. St. Louis, MO: Elsevier; 2017:1156-1157.
- Foster RA. Male Genital system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 3 6th ed. St. Louis, MO: Elsevier; 2016:468-471.
- Kim K-S, Kim O. A hermaphrodite dog with bilateral ovotestes and pyometra. J Vet Sci. 2006;7(1):87-88.
- Kobayashi K, Fujiwara T, Adachi T, Asahina M, Sasaki Y, Matsuda A, Nishimura T, Inui T, Kitamura K. Bilateral ovotestes in a female beagle dog. J Toxicol Pathol. 2007;20:111-115.
- Meyers-Wallen VN. Gonadal and sex differentiation abnormalities of dogs and cats. Sex Dev. 2012;6:46-60.
- Olivares A, Avila-Poveda OH. An ovotestis event in the gonochoric sea urchin Loxechinus albus (Echinodermata: Echinoidea). Brazilian Journal of Biology. 2019;79(3):548-551.
- Pérez-Gutiérrez JF, Monteagudo LV, Rodriguez-Bertos A, Garcia-Perez E, Sánchez-Calabuig MJ, García-Botey C, Whyte A, Sánchez de la Muela. Bilateral ovotestes in a 78, XX SRY-negative Beagle dog. J Am Anim Hosp Assoc. 2015;51(4):267-271.
- Roy S, Chaki KK, Nag TC, Misra KK. Ultrastructure of ovotestis of young and adult pulmonate mollusk, Macrochlamys indica Benson, 1832. Journal of Microscopy and Ultrastructure. 2016;4(4):184-194.
- Schlafer DH, Foster RA. Pathology of the genital system of the nongravid female. In: Maxie MG, ed. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. Vol 3. 6th ed. St. Louis, MO: Elsevier; 2016:361-362.
- Song Y, Xu Y, Liang M, et al. CRISPR/Cas9-mediated mosaic mutation of SRY gene induces hermaphroditism in rabbits. Bioscience Reports. 2018;38(2): BSR20171490.
- Voisin AS, Kultz D, Silvestre F. Early-life exposure to the endocrine disruptor 17-a-ethinylestradiol induces delayed effects in adult brain, liver and ovotestis proteomes of a self-fertilizing fish. Journal of Proteomics. 2019;194:112-124.