CONFERENCE 10, CASE IV:
Signalment:
3-year-old female canine, Pit Bull mix
History:
Prior to an ovariohysterectomy, the patient was reported to have a 4-month history of unexplained weight loss. Preoperative examination revealed a large intra-abdominal mass suspected to be associated with the spleennd a subcutaneous perivulvar mass. An enlarged, cavitated spleen, a small mass noted on liver, and perivulvar subcutaneous mass were submitted for histopathology.
Gross Pathology:
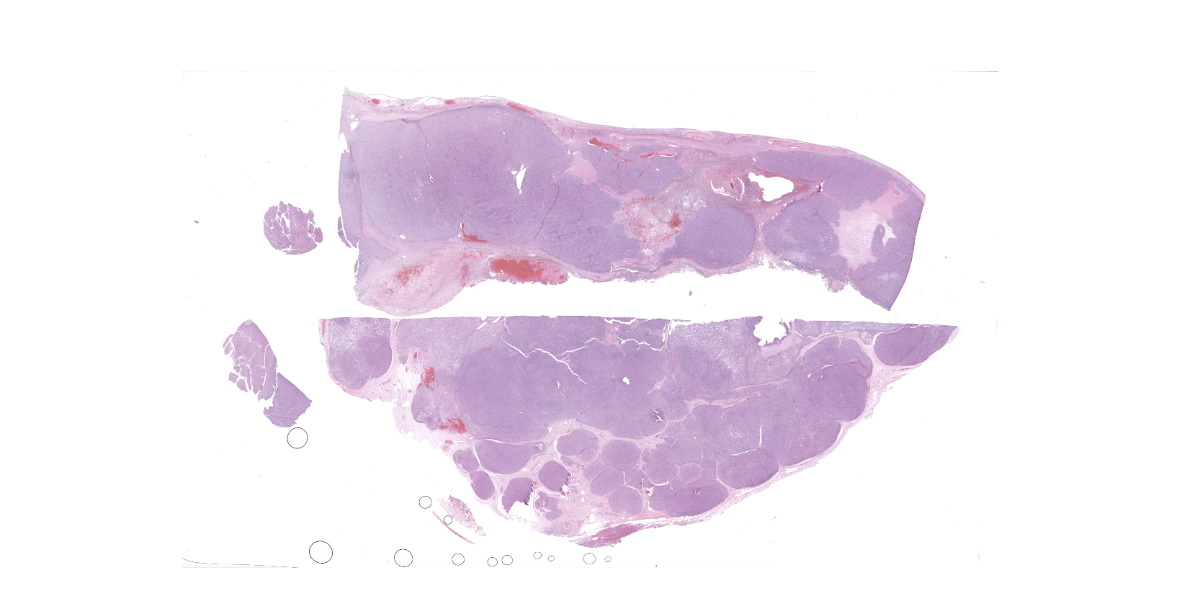
Approximately 80% of splenic parenchyma was severely enlarged with multiple white to tan, irregular, soft coalescing nodules and necrotic cavitated areas. The liver had a focal, less than 0.5cm in diameter, tan irregular nodule. The perivulvar subcutis mass was tubular, tan, and soft and approximately 4-5cm in diameter.
Laboratory Results:
Cytology identified a spindloid neoplastic population with microvesiculated cytoplasm in both the perivulvar subcutaneous mass and rare to occasionally in the splenic mass. These microvesiculated neoplastic cells were reminiscent of liposarcoma, but an epithelioid neoplasm could not be excluded.
Microscopic Description:
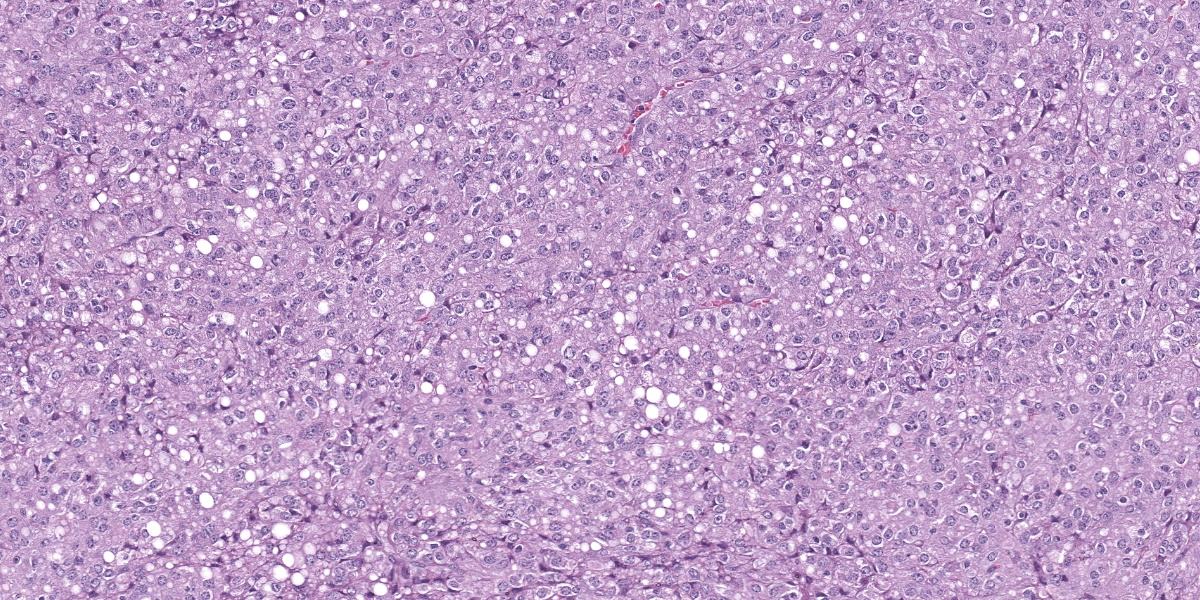
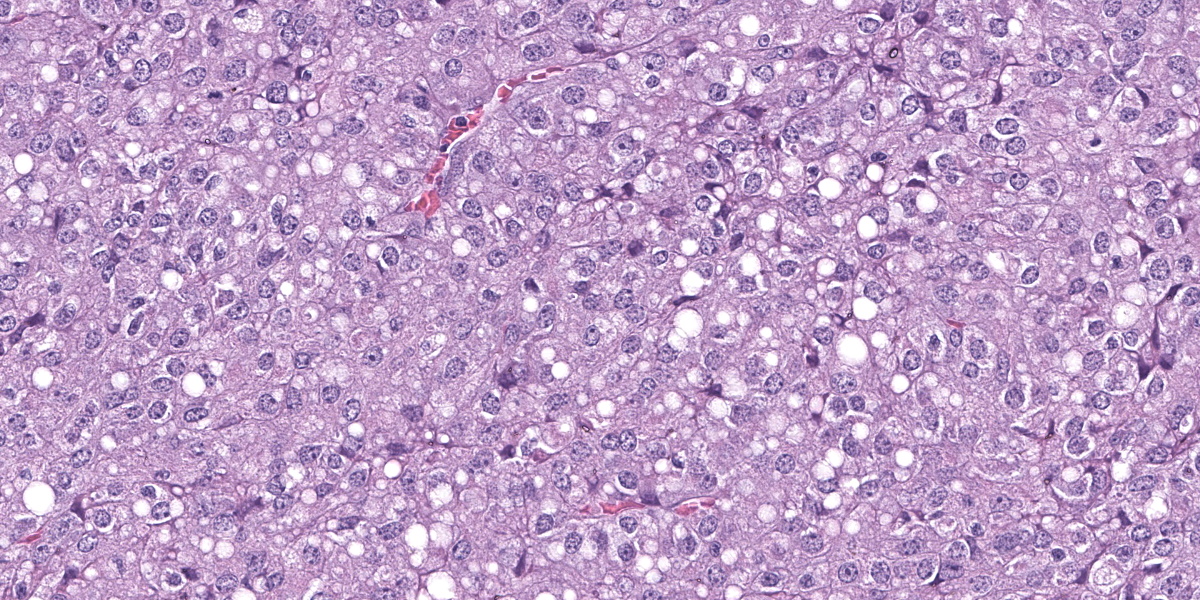
Splenic mass: Splenic parenchyma is expanded and replaced by multifocal to coalescing, poorly demarcated nodules of a moderately cellular neoplasm composed of pleomorphic cells arranged in nests and bundles separated by fine to moderate fibrovascular stroma. Neoplastic cells have indistinct cellular borders, moderate amounts of amphophilic cytoplasm that frequently contain variably sized clear discrete vacuoles (lipid), with one round to ovoid nuclei, with coarse chromatin and 1-3 discrete nucleoli. There is moderate anisocytosis and anisokaryosis with 5 mitotic figures in 10 high power fields (2.37mm2). There are multifocal areas of necrosis with fibrin and hemorrhage, multinucleated giant cells, macrophages with intracytoplasmic dark brown pigment or yellow granular pigment (heme). Remaining spleen is congested with increased plasma cells.
Hepatic mass, incisional biopsy. Replacing approximately 50% of section is an unencapsulated, moderately well demarcated, moderately cellular neoplasm of similar neoplastic cells as described in spleen. Sometimes, there are intracytoplasmic pink globules within cytoplasm of neoplastic cells.
Perivulvar subcutaneous mass: Mass is composed of multiple coalescing nodules of previously described neoplastic cells in spleen separated by moderate amounts of mature collagen interrupted by macrophages laden with brown globular pigment (hemosiderin), lymphocytes, plasma cells, and mineral.There is electrocautery artifact along periphery.
Immunohistochemistry/Special Stains:
Hepatic, perivular, and splenic masses are examined and have the same results as below.
- Cytokeratin: Neoplastic cells do not stain positively.
- Vimentin: Neoplastic cells have positive cytoplasmic staining.
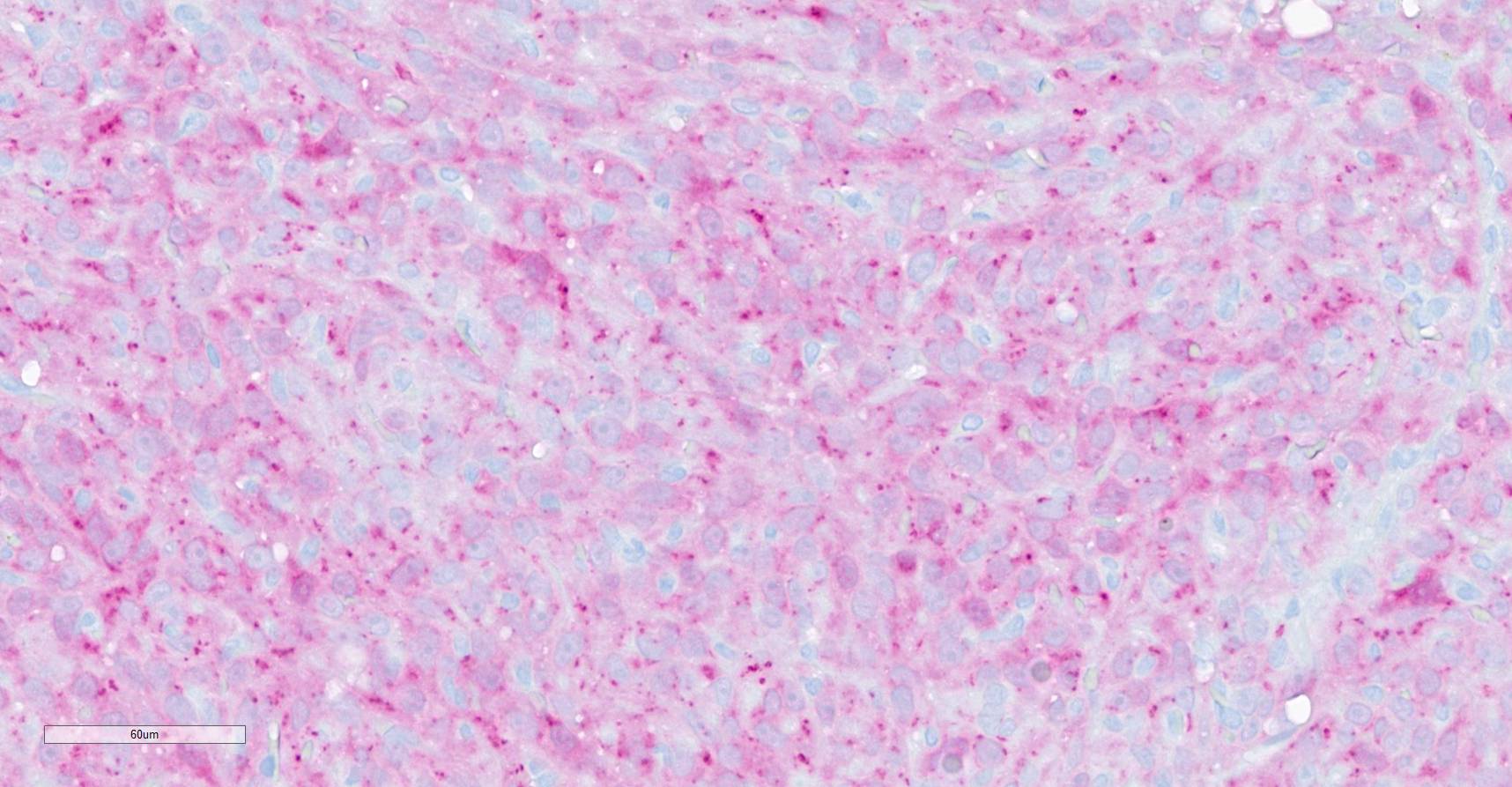
- Melan-A: Neoplastic cells exhibit weak cytoplasmic staining which are occasionally stained strongly in cytoplasm. No membranous staining is seen in the neoplastic cells.
- PNL-2: Approximately 10% of neoplastic cells exhibit cytoplasmic, occasionally membranous staining.
- CD18: Neoplastic cells do not stain positively.
- S-100: Neoplastic cells do not stain positively.
- Synaptophysin: Neoplastic cells do not stain positively.
- Chromogranin A: The cytoplasm of neoplastic cells diffusely are stained
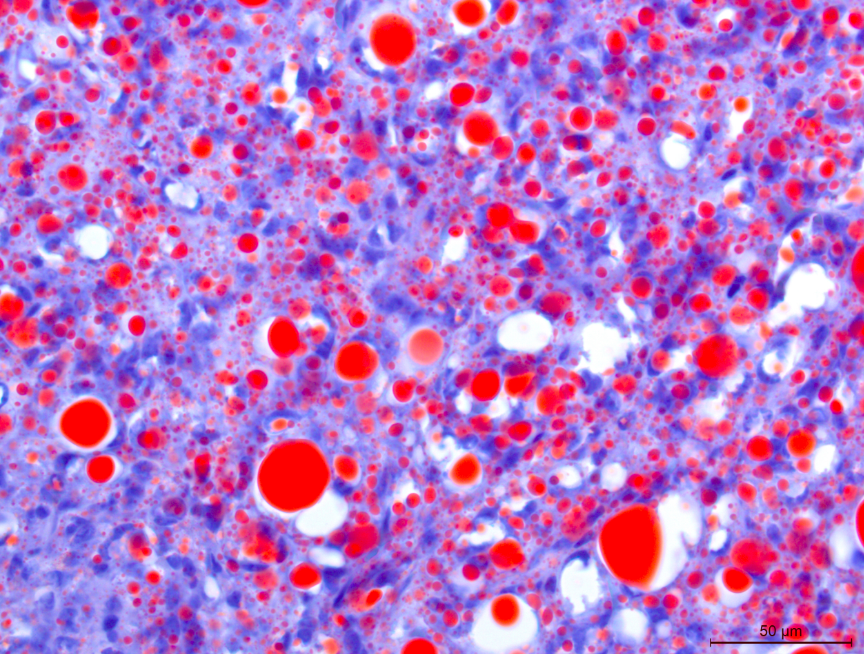
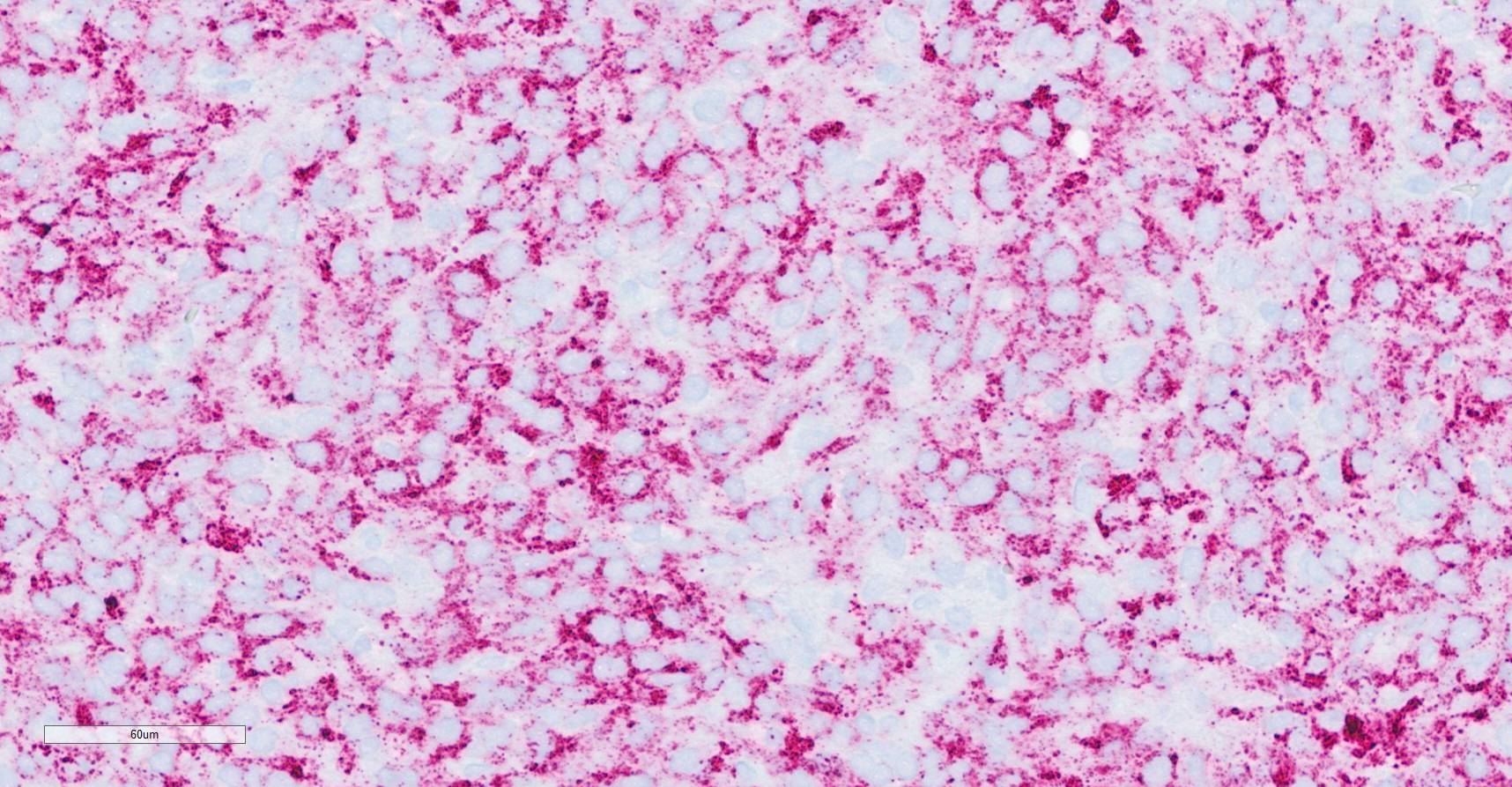
weakly which are considered negative. - Oil Red O: Neoplastic cells and vacuoles have positive intracytoplasmic staining.
Contributor’s Morphologic Diagnosis:
Spleen: liposarcoma with metastasis to perivulvar subcutis and liver
Contributor’s Comment:
Liposarcoma is an uncommon mesenchymal tumor originating from lipoblasts and lipocytes. More commonly, liposarcomas are a subtype of soft tissue sarcomas associated with the skin and subcutaneous tissues. Typically, locally invasive with low metastatic rates, liposarcomas in humans are classified by the World Health Organization into four histological subtypes: well differentiated liposarcoma/atypical lipomatous tumor, dedifferentiated liposarcoma, myxoid/round cell liposarcoma, and pleomorphic liposarcoma.1 In domestic animals, there are three subtypes of liposarcomas: well-differentiated, pleomorphic, and myxoid.4 In well differentiated liposarcomas, the majority of neoplastic cells have intracytoplasmic clear vacuoles.4 Dedifferentiated liposarcomas arise in association with well differentiated liposarcomas and can have morphologic overlap with pleomorphic liposarcomas that may be multinucleated and irregular with a few to rare neoplastic cells containing intracytoplasmic lipid vacuoles. 4 Myxoid liposarcomas are distinguished by an abundant myxomatous matrix.4
Visceral liposarcomas associated with the spleen are a subtype of non-angiomatous non-lymphomatous mesenchymal neoplasm with higher metastatic potential.3 Non-angiomatous non-lymphomatous mesenchymal neoplasms constitute 23-34% of primary splenic neoplasms and include fibrosarcoma, leiomyosarcoma, undifferentiated sarcoma, and liposarcoma.2 A study analyzing the outcome of 32 cases of splenic stromal sarcomas in dogs, identified that a mitotic count exceeding 9 mitoses per 10 high power fields (2.37mm2) to be a significant predictor of metastasis.2 While liver metastases have been reported with splenic liposarcoma, metastases to subcutis have not been reported.5
In this case, given the similarity between neoplastic cells in the liver, subcutis, spleen, and the significantly larger neoplastic mass within spleen, it is favored that the splenic liposarcoma metastasized to both the liver and subcutis. Alternatively, it is possible the subcutaneous liposarcoma and spleen occurred independently. Metastases of sarcomas to subcutaneous tissues is extremely rare in all species. A retrospective study performed in human medicine found less than 0.25% of patients with sarcomas had cutaneous metastases.10 Leiomyosarcomas were documented to be the most common mesenchymal tumor to metastasize to the skin.10
Generally, splenic sarcomas require additional immunohistochemistry or special stains for a definitive diagnosis. Specifically, particularly with a pleomorphic subtype of liposarcoma, Oil Red O is an invaluable stain for lipid within neoplastic cells.9 Oil Red O is typically performed on frozen tissue sections, though there is a protocol for staining formalin fixed sections. Another lipid stain performed on frozen tissue is Sudan black.9 Other immunohistochemical stains that may aid in favor of liposarcoma include S-100 and perilipin.8 In human liposarcomas, immunohistochemical markers include MDM2 (murine double minute 2) and CDK4 (cyclin dependent kinase 4) that have also been used in subtype classification.5
Initially, this case was tentatively diagnosed as a melanoma given the positive immunoreactivity to Melan-A. However, given the predominantly negative immunoreactivity to PNL2, this diagnosis was seemingly refuted. In general, PNL2 is more sensitive than Melan-A with less cross reactivity to nonmelanocytic neoplasms.6
Contributing Institution:
https://clinics.midwestern.edu/animal-health-institute/diagnostic-pathology-center
JPC Diagnosis:
Spleen (per contributor) and liver: Malignant neoplasm
JPC Comment:
The final case for this conference is a story within a story! When we first received this case, we considered the contributor’s diagnosis of liposarcoma based on both the gross and H&E features which were certainly suggestive. Although we ultimately reached a different final diagnosis in conference, the case was chosen as liposarcoma is not a common WSC submission.
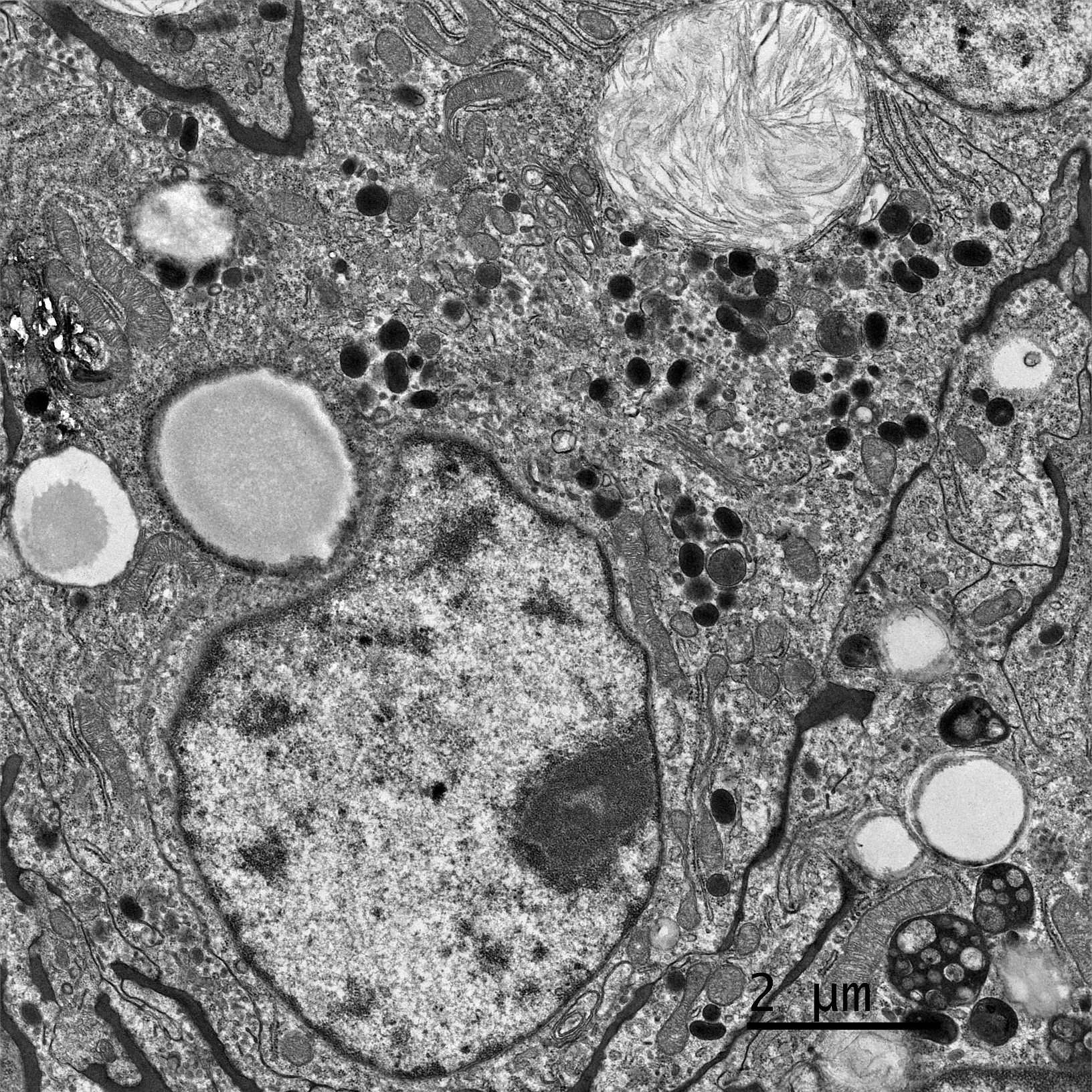
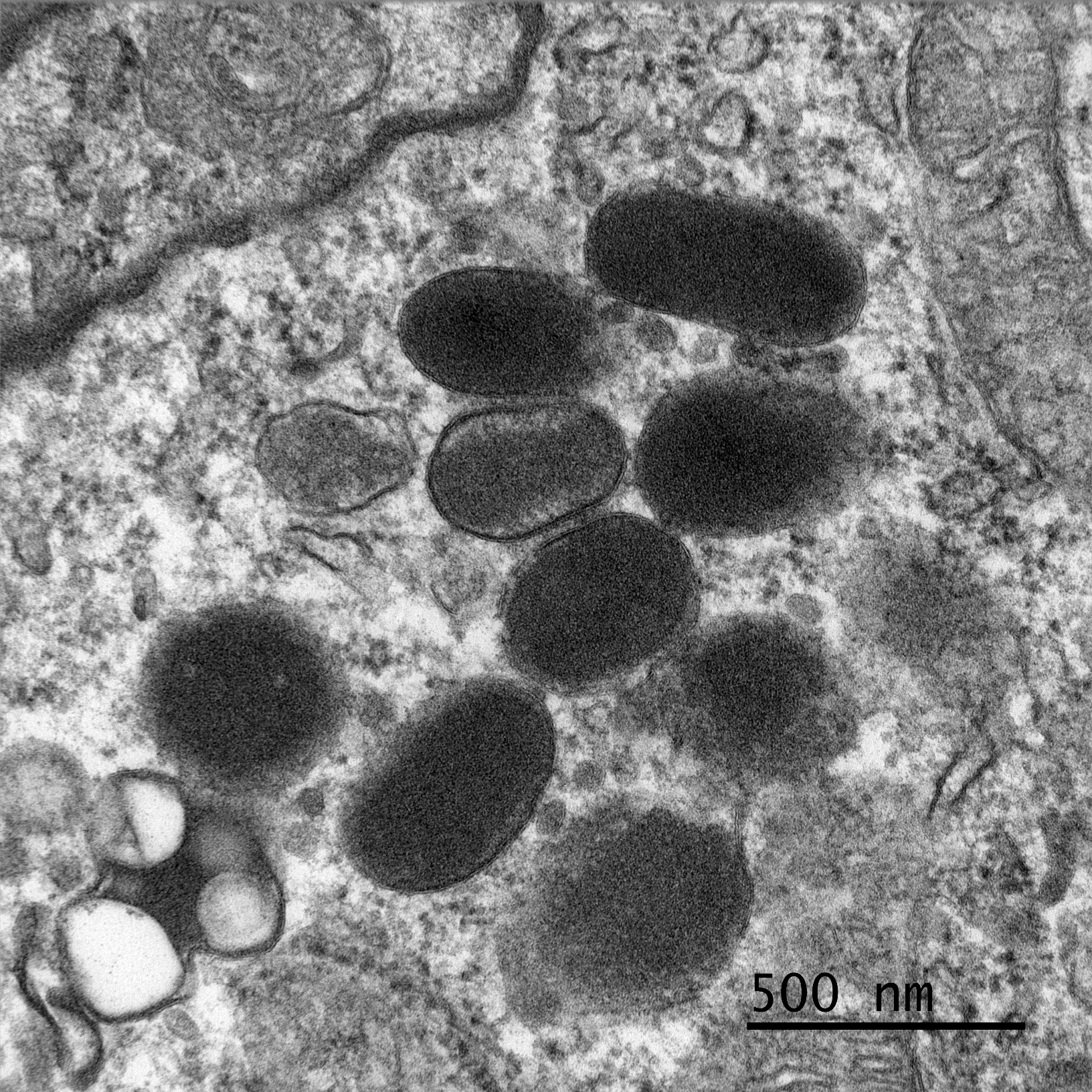
In prepping this case for conference, we noted that neoplastic cells were reactive for both Melan-A and PNL2 in our lab which did not fit with a liposarcoma. By chance, the contributor also consulted with Dr. Smedley on this case who repeated a melanocytic cocktail (Melan-A, PNL2, TRP-1, TRP-2) and performed a SOX10. The cocktail did label a proportion of cells (and we were cautioned by Dr. Smedley that these particular stains usually only stain a portion of the neoplastic cells. The SOX-10 was negative (which is unusual for melanomas, but the positive cells on the melanoma cocktail was de facie evidence that the tumor was indeed a melanoma.) As a result of these finding the contributor submitted this case to a third institution for transmission electronic microscopy where rare melanosomes within neoplastic cells. Given that Dr. Smedley, a renowned authority of canine melanoma) was on the WSC schedule this year, we knew that we couldn’t pass up an opportunity to revisit this case and the superficially conflicting results.
Dr. Smedley focused on differentiating this case from a poorly differentiated sarcoma. Oil Red O in this case highlighted lipid-rich portions of the neoplasm. Lipid within neoplastic cells was also noted on EM. While seemingly an odd finding, the “balloon cell” subtype of melanoma may contain lipid, glycogen, resulting in the clear swollen cytoplasm that gives this tumor its name. Finally, conference participants also considered sebaceous carcinoma as another rule out for lipid-rich tumors.
References:
- Amer KM, Congiusta DV, Thomson JE et al. Epidemiology and survival of liposarcoma and its subtypes: A dual database analysis. J Clin Orthop Trauma. 2020 Jul;11(Suppl 4):S479-S484.
- Ferrari R, Marconato L, Boracchi P et al. Splenic stromal sarcomas in dogs: Outcome and clinicopathological prognostic factors in 32 cases. Vet Comp Oncol. 2024 Mar;22(1):12-21.
- Gower KL, Liptak JM, Culp WT, Bravo L, Powers B, Withrow SJ. Splenic liposarcoma in dogs: 13 cases (2002-2012). J Am Vet Med Assoc. 2015 Dec 15;247(12):1404-7.
- Meuten DJ, Hendrick M. Mesenchymal Tumors of the Skin and Soft Tissues. In: Meuten DJ, ed: Tumors in Domestic Animals.5th edition. Ames, IO: John Wiley & Sons, Inc. 2017; 640.
- Nishikawa G, Minamiguchi S, Hata H et al. Dedifferentiated liposarcoma involving the spleen and splenic hilum: a report of a case with a rare growth pattern. Int Surg. 2015 Jan;100(1):128-32.
- Ramos-Vara JA, Miller MA. Immunohistochemical identification of canine melanocytic neoplasms with antibodies to melanocytic antigen PNL2 and tyrosinase: comparison with Melan A. Vet Pathol. 2011 Mar;48(2):443-50.
- Saik JE, Diters RW, Wortman JA. Metastasis of a well-differentiated liposarcoma in a dog and a note on nomenclature of fatty tumours. J Comp Pathol. 1987 May;97(3):369-73.
- Straub BK, Witzel HR, Pawella LM et al. Perilipin 1 Expression Differentiates Liposarcoma from Other Types of Soft Tissue Sarcoma. Am J Pathol. 2019 Aug;189(8):1547-1558.
- Tracy RE, Walia P. A method to fix lipids for staining fat embolism in paraffin sections. Histopathology. 2002 Jul;41(1):75-9.
- Wang WL, Bones-Valentin RA, Prieto VG et al. Sarcoma metastases to the skin. Cancer. 2012;118: 2900-2904.