Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 5
Sept 26th, 2018
CASE I: 1505184 (JPC 4066664-00).
Signalment: 14-month-old Holstein heifer.
History: Two 14-month-old Holstein heifers were presented to the large animal clinic as representatives from a heard outbreak, which affected five other heifers on the farm who had more severe signs and had subsequently died or were euthanized. This heifer (heifer A) died immediately prior to transit to the clinic, and had no known clinical signs. Her live herdmate (heifer B) had a 2-week-duration of clinical signs of unthriftiness, oral and cutaneous ulcers with ptyalism, and ocular signs including squinting, epiphora, and corneal ulcers. On physical exam, this heifer B was tachycardic and tachypneic with a rectal temperature of 103° Fahrenheit and generalized peripheral lymphadenomegaly. Heifers involved in the outbreak were also reported to have had cloudy eyes and appeared to be blind. Also housed in close physical proximity to the heifer herd were 5 sheep that had recently lambed. No treatments were performed at this time, and due to suspicion for malignant catarrhal fever, the live herdmate was euthanized without further clinical diagnostics or treatment.
Gross Pathology: Autopsy of the euthanized herdmate (heifer B) revealed severe crusting and ulcerative dermatitis and stomatitis including the following locations: perineal/perivulvar, periocular, mammary, perinasal/perioral and tongue. In addition, large raised to crateriform ulcers with adherent diphtheritic membranes were present throughout the rumen, as well as generalized lymphadenopathy, splenomegaly and corneal edema. Autopsy of the spontaneously dead heifer (heifer A) revealed meningeal edema and hyperemia with cerebellar coning, consistent with cerebellar herniation. The brain did not autofluoresce under exposure to ultraviolet light.
Laboratory results: A panel including serology and PCR tests was submitted on blood drawn from the live heifer (heifer B) immediately prior to euthanasia. A nested PCR test was positive for ovine herpesvirus-2 malignant catarrhal fever (MCF) virus, and negative for the alcelaphine herpesvirus-1 and 2 MCF viruses. Coronavirus PCR tests performed on intestinal contents were also negative. ELISA serology tests detected antibodies for bluetongue virus; however, PCR tests performed on serum for bluetongue virus were negative. No serum antibodies were detected by ELISA for foot and mouth disease virus, vesicular stomatitis virus (Indiana or New Jersey strains) or epizootic hemorrhagic disease of deer virus. Virus isolation performed on fresh lung, liver, kidney and spleen sections harvested immediately postmortem were negative for bovine viral diarrhea virus (BVDV) and infectious bovine rhinotracheitis (IBR) virus.
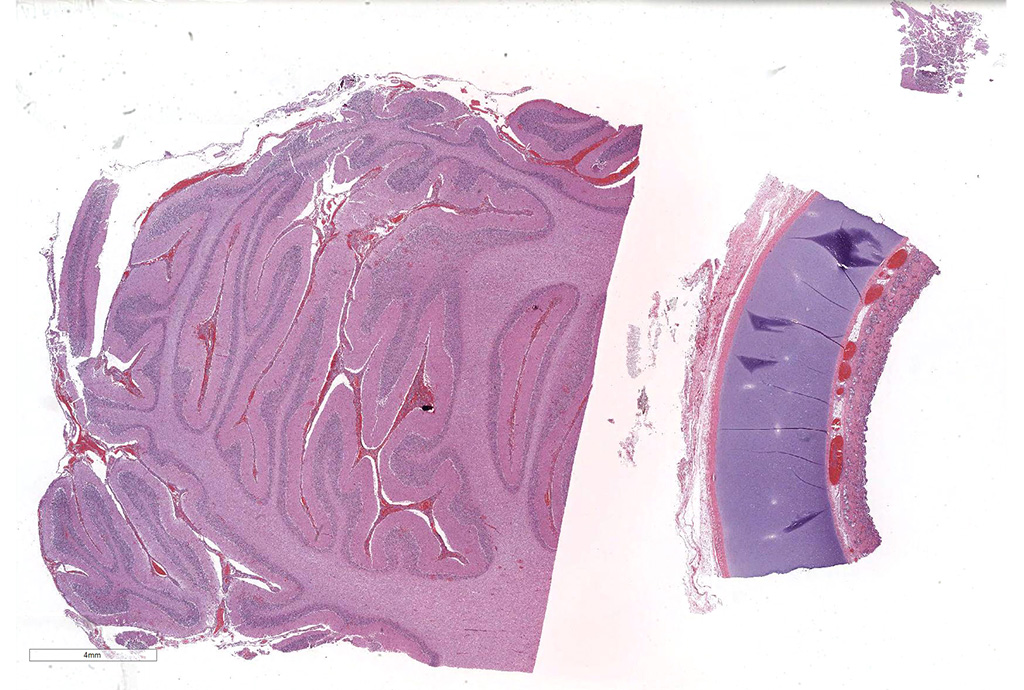
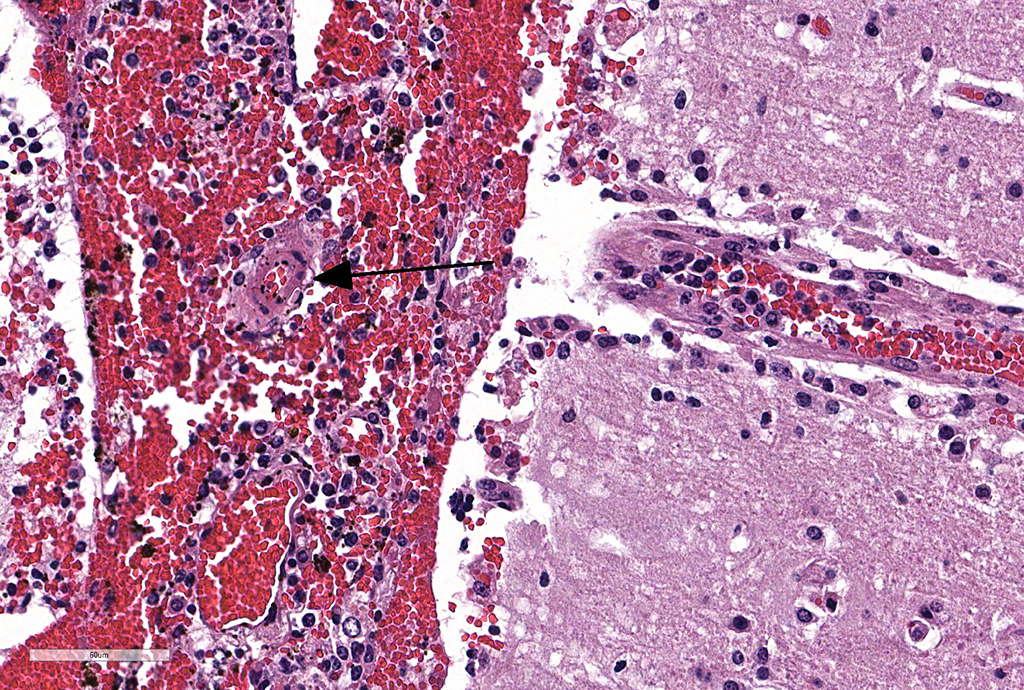
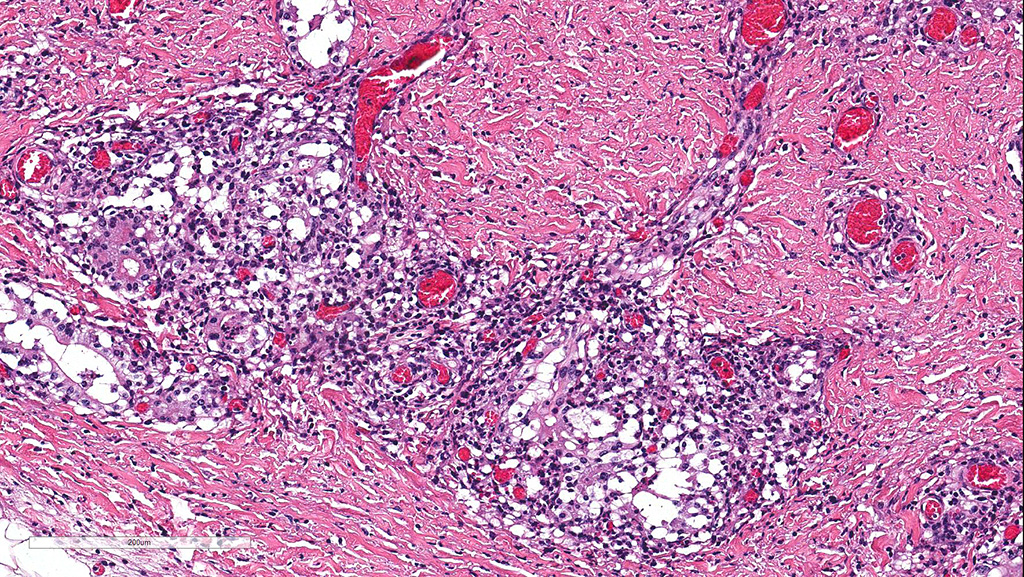
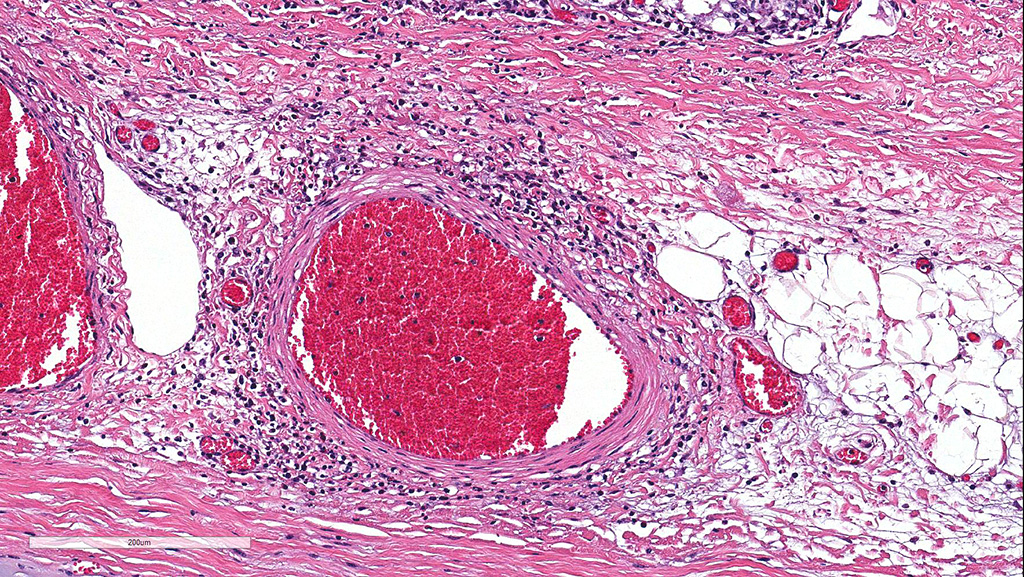
Microscopic Description: Cerebellum, Trachea (heifer A): Within the cerebellar leptomeninges and tracheal mucosa and submucosa, there is marked vasculitis with edema, multifocal to coalescing lymphohistiocytic infiltrates and hemorrhagic foci. The vasculitis is characterized by moderate numbers of lymphocytes, histiocytes, plasma cells and neutrophils that expand the tunica adventitia and transmigrate through the walls of varying caliber arterioles and venules. Segments of vascular walls demonstrate pyknosis and karyorrhexis with fibrinoid necrosis and hemorrhagic foci that are also scattered within the cerebellar parenchyma.
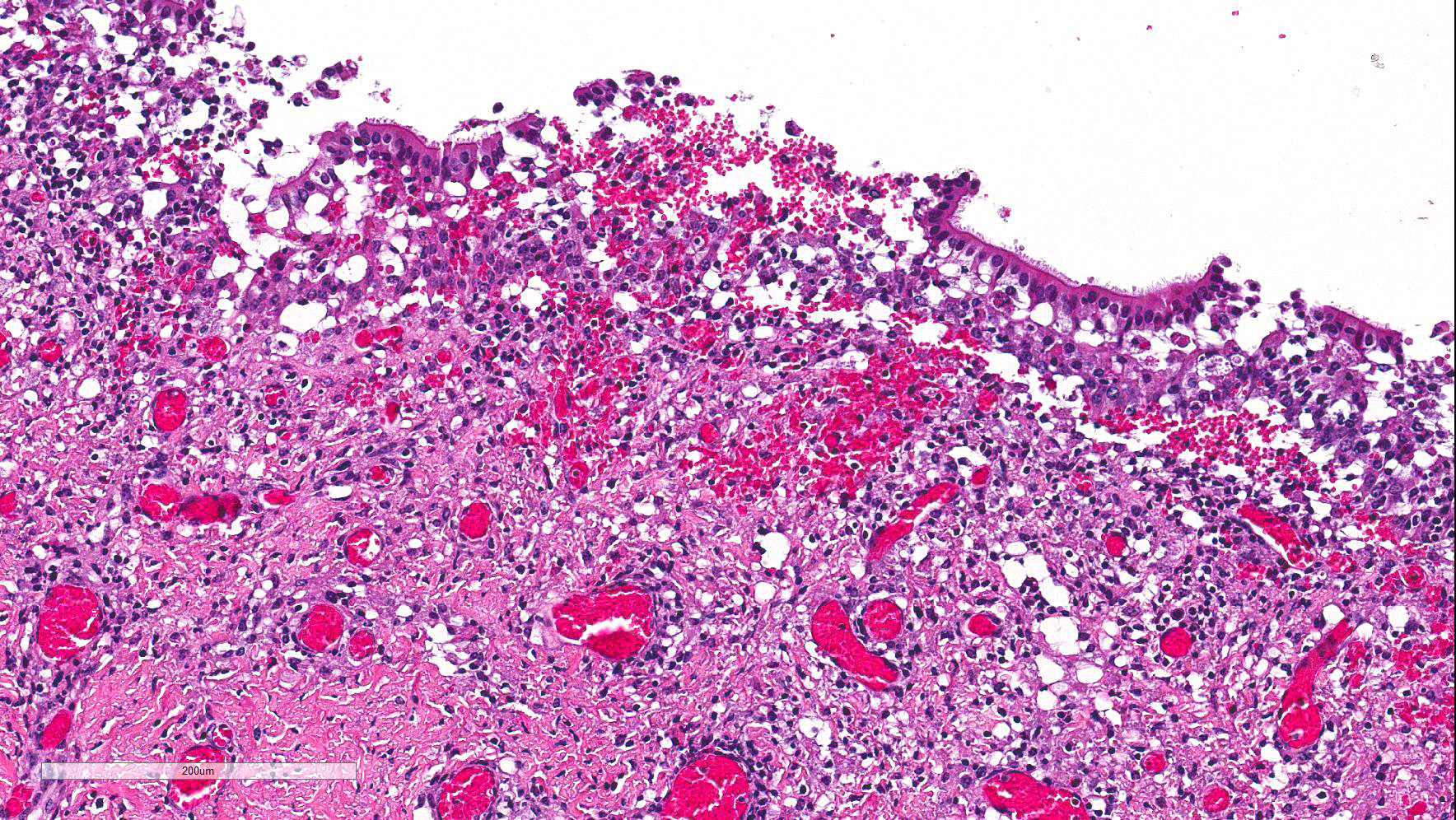
Numerous discrete clear vacuoles surround Purkinje cells, which often demonstrate cytoplasmic hypereosinophilia (degeneration). The tracheal mucosal epithelium is multifocally attenuated, with loss of apical cilia, or necrotic and denuded and replaced by eosinophilic and karyorrhectic debris, necrotic neutrophils and extravasated erythrocytes. Similar inflammatory populations surround and infiltrate seromucinous glands.
The kidney and urinary bladder from heifer A had similar vascular changes as described above in addition to multifocal associated interstitial inflammation with tubular necrosis and regeneration in the kidneys, and ulceration of the transitional epithelium of the urinary bladder with submucosal hemorrhage. Similar vascular changes and inflammatory infiltrates were also present in the cutaneous, oral and rumen lesions of heifer B, and the choroid of both eyes. In skin and mucosal lesions, inflammatory infiltrates often extended from the submucosa and dermis into the overlying epidermis/epithelium, occasionally blurring the basement membrane. The epidermis and squamous mucosa of the tongue and rumen frequently contained deep erosions to ulcers covered by waves of serocellular crusts containing colonies of bacteria, grit and fragments of plant material. In other regions there was multifocal keratinocyte necrosis within all layers of the epidermis/epithelium characterized by cytoplasmic hypereosinophilia with loss of intercellular desmosomes and pyknosis. Marked paracortical hyperplasia was observed within enlarged peripheral lymph nodes of heifer B.
Contributor’s Morphologic Diagnoses: Cerebellum: Moderate-severe lymphohistiocytic meningitis and segmental necrotizing vasculitis with multifocal acute-subacute hemorrhage.
Trachea: Moderate multifocal acute erosive and ulcerative tracheitis with severe lymphohistiocytic necrotizing vasculitis, perivasculitis and adenitis.
Contributor’s Comment: Malignant catarrhal fever (MCF) is a highly fatal lymphoproliferative viral disease reported worldwide affecting many wild and captive species of the order Artiodactyla.5,9 The MCF virus group comprises ten gammaherpesviruses, belonging to the recently assigned Macavirus genus4,6,7, six of which are naturally pathogenic.5,8 These include alcelaphine herpesvirus-1 (AIHV-1), alcelaphine herpesvirus-2 (AIHV-2), ovine herpesvirus-2 (OvHV-2), caprine herpesvirus-2, (CpHV-2), MCF of white-tailed deer (Odocoileus virginianus) (MCFV-WTD), and ibex-MCFV. Experimental infection with hippotragine herpesvirus-1 (HipHV-1) in domestic rabbits (Oryctolagus cuniculus) is documented, but no natural infections have been reported.5,9 Non-pathologic members include: gemsbok-MCF, muskox-MCF, and aoudad-MCF, also named after their respective carrier species.5,9 Identified natural host species including wildebeest (Connochaetes taurinus), domestic sheep (Ovis aries), wild and domestic goats (Capra hircus) and roan antelope (Hippotragus equinus) are a source of infection for susceptible, poorly adapted species such as American bison (Bison bison), many cervids, and domestic cattle (Bos taurus).
Of paramount economic importance to those managing bison, deer, and exotic game farms, as well as zoological collections, are alcelaphine herpesvirus-1 (AIHV-1) and ovine herpesvirus-2 (OvHV-2), the causative agents of wildebeest-associated MCF (WA-MCF), and sheep-associated MCF (SA-MCF) respectively. Susceptibility to infection by pathogenic MCF viruses and development of disease in non-reservoir hosts varies.5 In general, Bali cattle/banteng (Bos javanicus), white-tailed deer (Odocoileus virginianus), Pere David's deer (Elaphurus davidianus), and American bison are highly susceptible to disease following infection; water buffalo (Bubalus bubalis), and many cervids are intermediately susceptible; domestic cattle and pigs (Sus scrofa) are mildly susceptible; and fallow deer (Dama dama) are resistant.5,9 Additionally, laboratory animals such as rabbits (Oryctolagus cuniculus) and hamsters (Mesocricetus auratus) are susceptible to experimental infection.5
The clinical signs associated with WA-MCF and SA-MCF are indistinguishable.5,9 In most cases, animals present with fever, depression, anorexia, and a constellation of clinical signs, termed the "head and eye" form, which includes: oral mucosal ulcers, ptyalism, oculonasal discharge, dyspnea, corneal edema, hypopyon and photophobia. Additionally, dysentery, profuse catarrhal and mucopurulent nasal discharge and generalized lymphadenopathy are characteristic of the intestinal form, and severe nasal mucosal inflammation and hemorrhagic gastroenteritis are characteristic of the peracute form. Hematuria, stranguria, and convulsions can also be seen.9 In cattle infected with OvHV-2, there is usually an acute onset of clinical signs, but experimental studies show that some animals may present with disease months after infection.9
Bison are 100 times more likely to become infected, and 10,000 times more susceptible to developing MCF than cattle5, but experimentally-induced MCF with OvHV-2 in bison yields milder clinical signs (oculonasal discharge, peripheral lymphadenopathy) and vascular lesions (arteritis, phlebitis) than in cattle.9 CpHV-2 harbored asymptomatically in wild and domestic goats infects many species of deer and manifests usually as chronic weight loss, dermatitis and alopecia.5 Goats also harbor OvHv-2, and may be the source of infection for white-tailed deer in MCFV-WTD, which is now known to affect other cervids such as the red brocket deer (Mazama americana).5 CpHV-2 has also been linked to a recent report of MCF in a captive pudu (Pudu puda). It is important to note that viral shedding of OvHV-2 and AIHV-1 does not occur in clinically infected susceptible hosts5,9, so an infected animal is not a threat to its herd mates.
Common histopathological findings include: mononuclear mucosal inflammation and necrosis in the gastrointestinal tract, respiratory tract and skin; lymphocytic arteritis/phlebitis and perivasculitis in several tissues; lymphoid hyperplasia (paracortical and interfollicular); and panophthalmitis. Other distinguishing histologic features of MCF in American bison are degeneration and apoptosis ofurothelium and hemorrhagic cystitis.9 In cervids, most notably sika deer (Cervus nippon), granulomatous hyperplastic dermatitis and mural to perforating folliculitis are also seen. Widespread necrosis, intracytoplasmic viral inclusions and formation of syncytia, typical of herpesviral infections, are not observed in MCF.
Differential diagnoses include: mucosa! disease/bovine viral diarrhea virus (BVDV), infectious bovine rhinotracheitis (IBR), bluetongue virus, epizootic hemorrhagic disease, vesicular stomatitis, foot and mouth disease, rinderpest, and photosensitization. In this case, the clinical history, gross and histologic lesions, and positive PCR and serology tests confirmed Ovine herpesviral-2 associated MCF. Ancillary diagnostic tests in conjunction with histologic lesions allowed rule-out of the other differential etiologies listed above. Although the heifer had antibodies against Bluetongue virus, the negative PCR test was most consistent of prior exposure to the virus, but not active infection. Definitive diagnosis requires a combination of clinical signs in conjunction with positive serological test and/or confirmation with virus DNA detection in fresh or fixed tissue. This is due to the fact that infected, highly susceptible hosts may have negative titers, and that low levels of OvHV-2 have been detected in clinically healthy cattle.5.9
Key similarities and differences in transmission between alcelaphine and ovine gammaherpesvirus infections in reservoir hosts are listed below.5,9
AIHV-1:
- Harbored in wilderbeest as lifelong asymptomatic infection.
- Vertical and horizontal transmission is possible, though most infections are transmitted horizontally.
- Newborn calves are infected and continuously shed virus via ocular and nasal secretions. Viral shedding declines after about 3 months. Outbreaks in susceptible hosts are usually a seasonal phenomenon, corresponding to calving season in reservoir hosts.
OvHV-2:
- Harbored in sheep ( and goats) as lifelong asymptomatic infection.
- Transmission via placenta or through milk/colostrum is rare, most infections transmitted horizontally.
- Lambs are born uninfected. Most are infected by 2 months of age from carrier adults. 6-9-month adolescents are responsible for most viral shedding predominantly via nasal secretions. Uninfected lambs remain susceptible to infection as adults.
- Sporadic outbreaks in susceptible hosts as well as seasonal outbreaks corresponding to calving season in carrier hosts.
Most of our understanding of MCF is derived from in vitro studies of AlHV-1, WA-MCF.5,12 Though OvHV-2 has yet to be cultured in vitro, the discovery that OvHV-2 viral particles are shed copiously in sheep nasal secretions has helped elucidate the pathogenesis of SA-MCF, and will be the focus of this discussion. We now know that the pathogenesis ofOvHV-2 infection in sheep (the natural host) involves a 3-stage replication cycle, characterized by entry and initial replication in the lung, maintenance with latent infection in lymphocytes, and exit via replication and shedding in nasal mucosal epithelium.5 This differs from the 2-stage replication cycle in susceptible hosts with only entry, and maintenance (with latent and lytic viral replication), and no viral shedding.5
In general, sheep are better adapted to controlling the initial respiratory infection with OvHV-2, as evidenced by a significant increase in the level of expression of multiple immune-related genes when compared to susceptible species like bison and rabbits, who seem to have a less effective initial immune response in the lung.5,9 The products of the following viral genes are used to determine infection patterns in OvHV-2: open reading frame (ORF) 25 (a major capsid protein associated with lytic viral replication), ORF73 (a latency-associated nuclear antigen), ORF50 (R-transactivator that induces latent to productive cell cycle switch), and ORF9 (a DNA polymerase)5,9
In sheep, lytic replication in the lower respiratory tract after initial infection is associated with detection of ORF25 transcripts. This is followed by a change in viral tropism from alveolar epithelial cells to lymphoid cells, and subsequent dissemination of latently infected T-lymphocytes. Although viral DNA may be detected in many tissues in sheep, ORF25 transcripts are rarely detected, indicating the prevalence of a latent stage in tissues in the natural host.5 Although mechanisms of reactivation are unclear, shedding following reactivation occurs in the nasal mucosal epithelium.5 Conversely, in MCF-susceptible species such as bison and rabbits infected with OvHV-2, dissemination occurs with a latent and lytic pattern of viral gene expression, with viral gene transcripts ORF25, 50 and 73 detected in multiple organs during MCF disease.5 Systemic viral lytic replication is positively associated with severe illness in OvHV-2 infection as evidenced in bison and rabbits with SAMCF.5 In contrast to OvHV-2, studies with AIHV-1 infection in rabbits (a susceptible species) indicate that the virus is not productive during MCF disease, with low expression of viral genes (< 10% of viral genome) and very low detection of viral particles in tissues.10 Palmeira et al. (2013) confirmed the expression of ORF73 (the latency-associated nuclear antigen) during active MCF disease, without detectable expression of other ORF genes necessary for viral production, giving supportive evidence that active WA-MCF disease occurs with a latent viral infection. These findings were further confirmed when experimental nebulization of rabbits with an ORF73- deleted recombinant virus, showed effective initial host infection without the development of MCF lesions. Since it was also demonstrated at this time that AIHV-1 ORF73-null viral infection elicits an antibody response, this null-virus is currently being explored as a potential candidatefor vaccine development.10
In susceptible host species, lesions are associated with multisystemic lymphohistiocytic vasculitis and vascular necrosis, producing multiorgan inflammation, hemorrhage and ulcers. It is controversial whether the lymphoproliferation that results in generalized lymphadenopathy and immunosuppression is a direct result of viral infection of T lymphocytes, or a triggered dysregulation of uninfected I- lymphocytes.5 In MCF disease, cytotoxic T lymphocytes are the incriminated cell type in vascular lesions.5,11 In OvHV-2, CD8+ perforin+ cytotoxic T cells as well as CD4+ perforin- regulatory T-cells and B-cells, are involved in vascular lesions.5,8 IL-10, TNFa, IFNy, and IL-4 are demonstrated, with no expression of IL-1~ and IL-2 in OvHV-2 cell lines.5 In AlHV-1, CD3+ CD8+ CD4-cytotoxic T-cells with increased expression of activated surface markers CD25 and CD44, high levels of IFNy, and perforin, and reduced levels IL-2 are characteristic.4,5 It appears that suppression of IL-2 production and signaling, and thus, suppression of immune regulation is common to the pathogenesis of disease in both ALHV-1 and OvHV-2 MCF infections.5
Interestingly, in a recent study of OvHV-2 in bison, cytoplasmic ORF25 protein was demonstrated in perivascular fibroblasts, with no detection in endothelium, vascular leiomyocytes or transmigrating leukocytes, presenting yet another potential avenue for the pathogenesis of vascular lesions in MCF.7
The lesions in these two heifers included a wide spectrum of clinical signs, as well as gross and histologic lesions seen with MCF disease, including cutaneous, gastrointestinal (tongue and rumen), urinary, respiratory, ocular and neurologic manifestations. This herd outbreak serves as a reminder of the various forms of MCF, the importance of biosecurity regarding separation of ruminant species in a production operation, and emphasizes the multiple modalities required to make a definitive etiologic diagnosis.
JPC Diagnosis: 1. Cerebellum: Vasculitis, necrotizing and lymphocytic, multifocal, moderate, with hemorrhage, neuronal necrosis, and diffuse lymphohistiocytic meningitis.
- Trachea: Vasculitis, necrotizing and lymphocytic, diffuse, moderate, with hemorrhage and mucosal and submucosal gland necrosis.
Conference Comment: The contributor has done an excellent job summarizing the condition knowns as malignant catarrhal fever, from species affected, basic pathogenesis, and molecular aspects of this disease.
American bison are considered one of the most sensitive species to the various viruses which may cause MCF, particularly that of OHV-2. Dr. Donal O’Toole has written and published extensively on this topic7,8,9, and his comments certainly bear summarization for this species, one of the U.S. most majestic symbols.
Bison with MCF due to OvHV-2 are most often found dead or dying, due to the large size of bison pastures and the inherent difficulties in patrolling these large areas of land. Mortality among bison heard approaches 100%, especially in those in feedlots. Some feedlots experience up to 75% of all deaths as a result of MCF. Aerosol transmission is an important route of exposure between bison in sheep, especially in sale barns.9
Acute clinical disease in bison predominates over subclinical and chronic forms. In natural outbreaks, preclinical pneumonia is inconspicuous or may be masked by aspiration pneumonia of the clinical disease. Characteristic gross findings in terminal animals include oculonasal discharge, hemorrhagic cystitis, necrohemorrhagic typhlocolitis, ulcerative rhinitis, stomatitis, pharyngitis, laryngitis, and esophagitis.9 Abortions may also occur. Due to its prevalence in affected animals and rarity in other conditions, hemorrhagic cystitis may be used by owners in making a provisional diagnosis of this condition. Generalized lymph node hyperplasia is usually present, but rarely as pronounced as in cattle. Ulceration in the forestomachs and alimentary tract is common. Traumatic lesions due to goring may be seen and may be incorrectly assumed to be the sole cause of illness.
Histologic lesions are similar to that discussed with cattle; in addition, degeneration of urothelium is a consistent and helpful feature in fresh carcasses. Fibrinoid necrosis is less common bison than in cattle. Myocardial necrosis may be seen in more longstanding cases as a result of exertional myopathy.9
Sampling for OHV-2 is occasionally problematic as the distribution of OHV-2 antigen may not necessarily reflect the pansystemic nature of disease. Sampling a minimum of 2 tissues, both lymphoid and nonlymphoid is recommended, and OHV-2 DNA may also be found in peripheral blood of clinically healthy bison. Interestingly in spite of the widespread and often severe lesions, no viral particles are detectable ultrastructurally, and the expression of viral antigens in tissue is limited. It is generally assumed that the small fraction of infected lymphocytes induces proliferation and deregulation of uninfected cells, which may induce lesions due to non-specific cytotoxic effects.9
Contributing Institution:
University of Pennsylvania, School of Veterinary Medicine, Department of Pathobiology
http://www.vet.upenn.edu/research/academic-departments/pathobiology/pathology-toxicology
References:
- Ackermann, M et al. Pathogenesis of gammaherpesvirus infections. Vet Micro 2006; 153(3): 211-222.
- Cunha, CW et al. Antibodies to ovine herpesvirus 2 glycoproteins decrease virus infectivity and prevent malignant catarrhal fever in rabbits. Vet Micro 2012; 175(2):349-355.
- Cunha, CW et al. Ovine herpesvirus 2 infection in American bison: virus and host dynamics in the development of sheep-associated malignant catarrhal fever Vet Micro 2012; 159(3):307-319.
- Dewals, B. "Malignant catarrhal fever induced by Alcelaphine herpesvirus I is characterized by an expansion of activated CD3 CD8 CD4-T cells expressing a cytotoxic phenotype in both lymphoid and non-lymphoid tissues." Vet Res 2011; 22:42-95.
- Li, Hong, et al. Malignant Catarrhal Fever: Inching Toward Understanding Ann Rev Anim Biosci 2014; 2(1):209-233.
- Modesto P et al. First report of malignant catarrhal fever in a captive pudu (Pudu pudu). Res Vet Sci 2015; 99: 212-214.
- Nelson, DD et al. Fibroblasts express OvHV-2 capsid protein in vasculitis lesions of American bison (Bison bison) with experimental sheep-associated malignant catarrhal fever. Vet Micro 2013; 166(3): 486-492.
- Nelson, DD et al. "D8+/perforin+/WCl-yo T cells, not CDS+ a~ T cells, infiltrate vasculitis lesions of American bison (Bison bison) with experimental sheep-associated malignant catarrhal fever." Vet Immunol and Immunopathol 2010; 136(3):284-291
- O'Toole, D, Li, H. The pathology of malignant catarrhal fever, with an emphasis on ovine herpesvirus-2. Vet Pathol 2014; 51(2):437-452.
- Palmeira L et al. "An essential role for y-herpesvirus latency-associated nuclear antigen homolog in an acute lymphoproliferative disease of cattle. Proc Nat Acad Sci 2013; 110(21): El933-El942.
- Simon S, et al. The vascular lesions of a cow and bison with sheep-associated malignant catarrhal fever contain ovine herpesvirus 2-infected CDS+ T lymphocytes. J Gen Virol 2003; 84(8):2009-2013.
- Taus, NS et al. Malignant catarrhal fever in American bison (Bison bison)experimentally infected with alcelaphine herpesvirus 2." Vet Microbiol 2014; 172(1):318-322.
- Vikoren T et al. A geographic cluster of malignant catarrhal fever in moose (Alces alces) in Norway. J Wildl Dis 2015; 51(2):471-474.