Signalment:
Adult male fox squirrel (
Sciurus niger)Over the course of several days, five
squirrels were found dead under a tree at a residence in Colorado.Prior to
death, all of the squirrels had similar clinical signs which included
hindquarter paralysis, lethargy, and heavy breathing.
Gross Description:
Two fox squirrels were presented for
postmortem examination in good body condition with minimal autolysis.No
evidence of trauma was identified.The adult male squirrel had no significant
gross lesions and stomach contents were within normal limits, including grainy
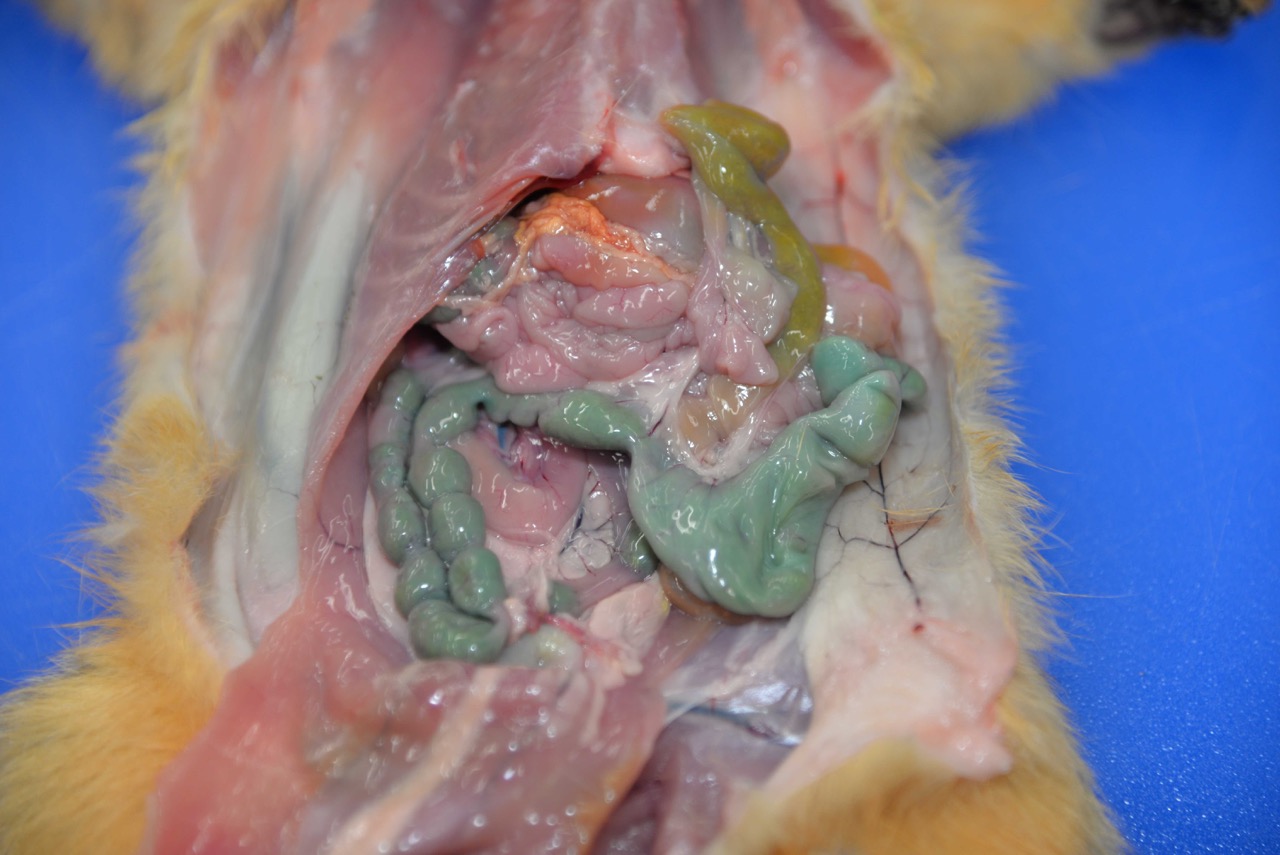
yellow-brown ingesta.The adult female had turquoise-green granular material
on the fur of the upper lip and similar material within the stomach.The
colonic contents were stained a distinct turquoise-green.
Histopathologic Description:
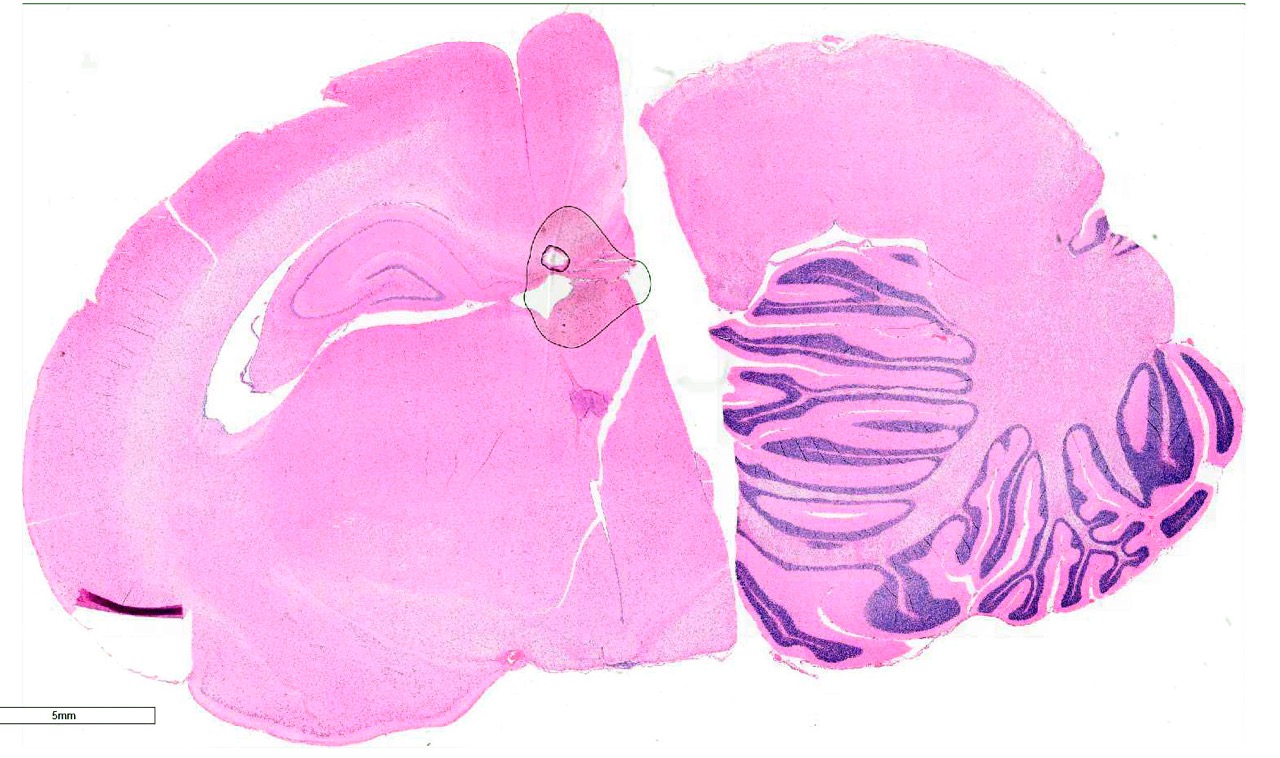
Brain, 2 sections (cerebellum and cerebral cortex including
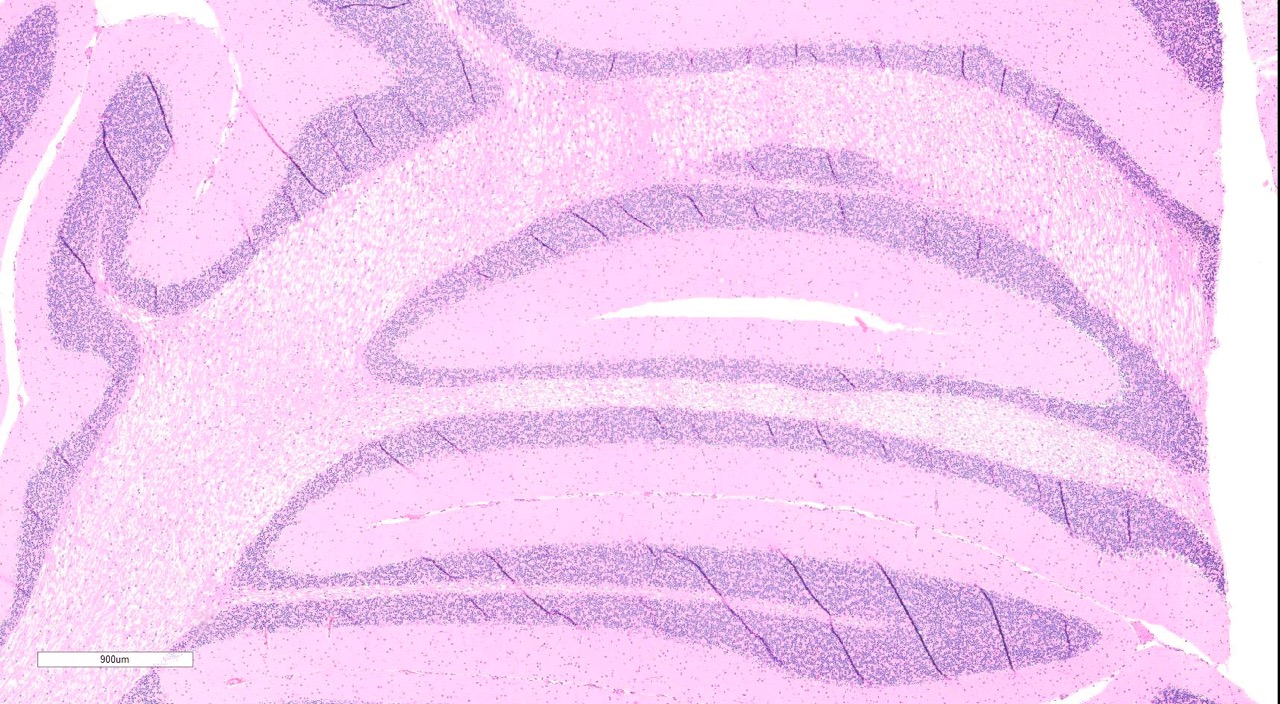
hippocampus): Diffusely throughout both sections, the white matter is
characterized by moderate to severe extracellular vacuolization.Vacuoles are
formed by variably swollen myelin sheaths which occasionally coalesce into
large extracellular clear spaces.Dilated myelin sheaths contain normal to
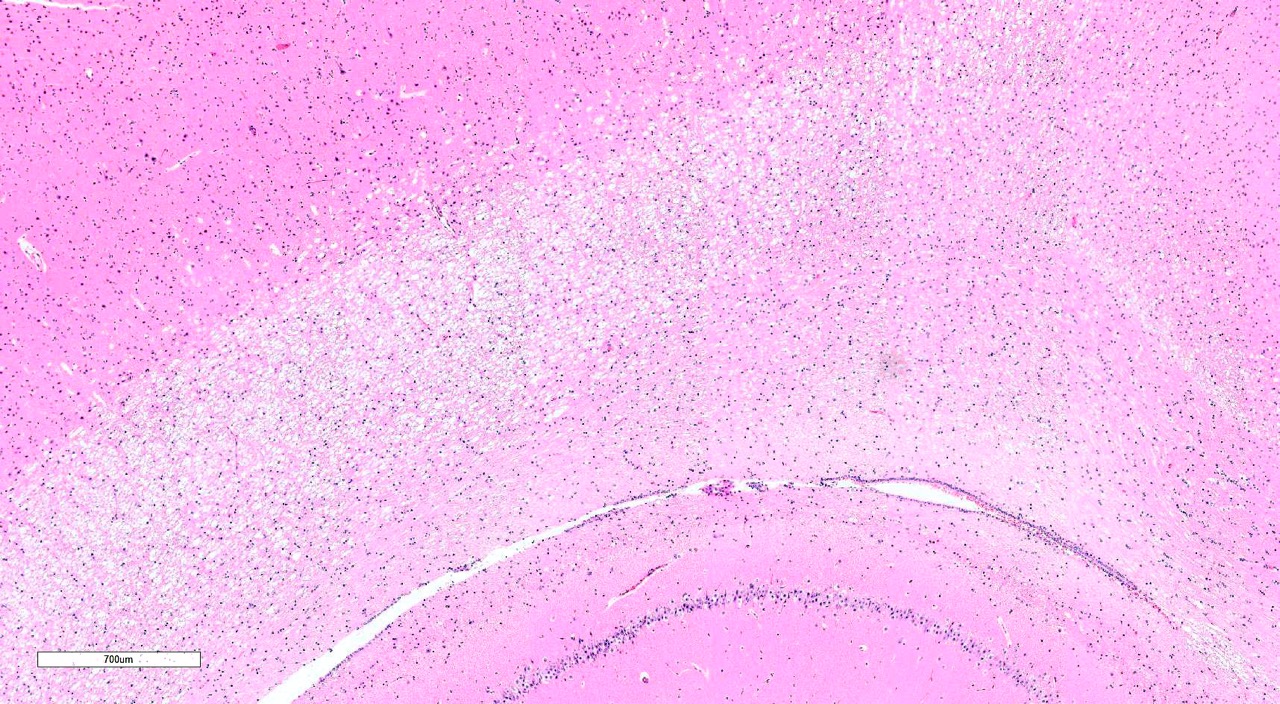
minimally swollen axons.Scattered throughout the grey matter of the cerebral
cortex and rarely within the hippocampus are low numbers of neurons with
degenerative changes, including central chromatolysis and occasional pyknosis.
Rare neurons are shrunken, angular, and hypereosinophilic with karyolysis
(necrosis).Clefts within the perikaryon of multiple neuronal cell bodies are
consistent with fixation artifact.
Morphologic Diagnosis:
Cerebellum
and cerebral cortex: Vacuolar myelinopathy, severe, diffuse, with mild,
multifocal neuronal degeneration and necrosis.
Lab Results:
Adipose tissue contained
desmethylbromethalin.
Condition:
Bromethalin toxicosis, squirrel
Contributor Comment:
Bromethalin
toxicosis was strongly suspected based on the history and collective gross and
histologic findings.This suspicion was confirmed by the presence of desmethylbromethalin
in the adipose tissue.Desmethylbromethalin is a toxic metabolite of
bromethalin, a potent neurotoxin and the active ingredient in a variety of
rodenticides.The mechanism of action involves un-coupling of oxidative
phosphorylation, resulting in decreased ATP production and diminished Na+/K+
pump activity.
9,10 In the CNS, the net result is severe, acute
fluid retention and a dramatic elevation in cerebrospinal fluid pressure.
Bromethalin is metabolized to desmethylbromethalin th-rough N-demethylation by
hepatic mixed-function oxygenases and is excreted predominantly in the bile.
The oral LD
50 is 2.38-5.6 mg/kg in the dog and 0.4-0.71 mg/kg in the
cat.
3,9 A relative resistance to toxicity has been demonstrated in
species unable to metabolize bromethalin to desmethylbromethalin (e.g. guinea
pigs with an LD
50 of 1000 mg/kg).
10 Short of chemical
confirmation, diagnosis of bromethalin toxicosis is based on likelihood of
exposure and development of corresponding clinical signs, including muscle
tremors, seizures, dypsnea, hyperexcitability, hind limb ataxia, and paresis to
paralysis.Severity and onset (2-
14
hours post-ingestion) are dose-dependent.
3 -
Bromethalin is
indistinguishable from anticoagulant rodenticides in appearance and color, and
gross lesions are uncommon.Diffuse white matter vacuolization is the
characteristic histologic lesion, and ultrastructural studies have demonstrated
intramyelinic vacuoles with separation and splitting of myelin lamellae.
4,5
Luxol fast blue-periodic acid Schiff stain has demonstrated myelin
displacement due to edema with no apparent net myelin loss.
5-
Hypertrophied astrocytes and oli-godendrocytes have also been reported.
5-
Vacuolization of the optic nerve occurs in most cases.
4,5 Similar
white matter vac-uolization is seen with triethyltin and hexachlorophene
neurotoxicosis.
7,8- -
Bromethalin use
has increased in recent years in association with new regulations prohibiting
residential use of second generation anticoagulant rodenticides.While
bromethalin remains readily available for over-the-counter sales, many
anticoagulant rodenticides with similar names (e.g. brodifacoum, bromadiolone)
have been removed.Thus, bromethalin toxicity is gaining in importance due to
increased popularity of neurotoxic rodenticides.
JPC Diagnosis:
Cerebrum
and cerebellum, white matter: Vacuolar myelinopathy, diffuse, severe.
Conference Comment:
Conference
participants discussed this lesion as being very -Ç-ÿquiet histologically, with
minimal if any response to the swelling of myelin sheaths. The moderator
discussed how this lesion contrasts with a demyelinating lesion, which
manifests histologically as a patchy, less diffuse distribution and with at
least some degree of glial response.In this example of bromethalin toxicity,
there is a distinct absence of swollen axons and spheroids, and the
oligodendrocytes appear quiescent.
The differential diagnosis
discussed by participants for a similar histologic lesion in other species
included other toxicants, such
as hexachlorophene and ammonia, as well as plant toxins seen in
various parts of the world, such as
Stypandra spp. in Australia and
Helichrysum
spp. in Africa.The lesions of bromethalin in the central nervous system of
these squirrels also bear resemblance to those of avian vacuolar myelinopathy,
which is seen in North America secondary to a cyanobacterial toxin that grows
on non-native aquatic vegetation.The condition is frequently lethal and
affects various avian species, such as bald eagles and American coots in the
southeastern United States.
6 Another cause of similar white matter
specific vacuolar change includes branched-chain alpha-ketoacid decarboxylase
de-ficiency (maple syrup urine disease) in cattle.
Despite
the apparent frequency with which bromethalin intoxication occurs in domestic
animals and wildlife species, there are surprisingly few reports in the recent
professional literature.In addition to the more acute syndrome discussed
above, a paralytic syndrome is also described when concentrations below the LD
50
are ingested; it includes ataxia, CNS depression, and paralysis which may
develop over a period of days and worsen over a period of weeks.
2-
The acute syndrome has also been reported to occur when smaller doses (below
the LD
50) are ingested in dogs and may relate to treatment with
activated charcoal, which can result in idiosyncratic hypernatremia in rare
cases. Dramatic changes in sodium levels can result in CNS associated clinical
signs and lesions, including cortical laminar necrosis in cases of salt
intoxication; conversely, osmotic demyelination can occur in cases where there
is a sudden increase in sodium in a hyponatremic animal. Histologic lesions in
osmotic demyelination and the changes seen in bromethalin toxicity can have similarities,
although in osmotic demyelination there is myelin and oligodendrocyte loss
which does not occur with bromethalin intoxication.
1
The green-tinged or turquoise
coloring seen in the gross image is characteristic of dyes used in certain
rodenticides, as well as in some fertilizers and pesticides,
2 which
can make confirmation of bromethalin intox-ication challenging.
1-
The highest con-centration of desmethylbromethalin is us-ually found in adipose
tissue, which is the most important tissue sample for diagnostic toxicology
testing in suspected cases of bromethalin intoxication.
2-
Bromethalin is not only lipid-soluble, but also readily crosses the blood brain
barrier.
1 Additionally, in cases of mild white matter vacuolation,
both light microscopy and ultrastructural examination may not be able to
precisely differentiate the changes of bromethalin intoxication from those of
autolysis, the latter of which are especially common in wildlife species.
2-
Neuron-specific nuclear protein (NeuN) -and
glial fibrillary acidic protein (GFAP) may
be useful in distinguishing subtle changes from autolytic artifact in
questionable cases.Decreased NeuN immunoreactivity can indicate neuronal loss
and/or metabolic stress and increased GFAP imm-unoreactivity is indicative of
reactive astrocytosis.
1
References:
1. Bates MC, Roady P, Lehner AF, Buchweitz JP, et al. Atypical bromethalin intoxication in a dog: pathologic features and identification of an isomeric breakdown product. BMC Vet Res. 2015; 11(244):1-9.
2. Bautista AC, Woods LW, Filigenzi MS, Puschner B. Bromethalin poisoning in a raccoon (Procyon lotor): diagnostic considerations and relevance to nontarget wildlife. J Vet Diagn Invest. 2014; 26(1):154-157.
3. Dorman, DC, Parker, AJ, Buck, WB. Bromethalin Toxicosis in the dogs. Part I: Clinical Effects. J Am Anim Hosp Assoc. 1990; 26(6): 589-594.
4. Dorman, DC, Simon, J, Harlin, KA, Buck, WB. Diagnosis of bromethalin toxicosis in the dog. J Vet Diagn Invest. 1990; 2:123-128.
5. Dorman, DC, Zachary, JF, Buck, WB. Neuropathologic findings of bromethalin toxicosis in the cat. Vet Pathol. 1992; 29:139-144.
6. Haynie RS, Bowerman WW, Williams SK, Morrison SK. Triploid grass carp susceptibility and potential for disease transfer when used to control aquatic vegetation in reservoirs with avian vacuolar myelinopathy. J Aquat Anim Health. 2013; 25(4):252-259.
7. Nakaue, HS, Dost, FN, Buhler, DR. Studies on the toxicity of hexachlorophene in the rat. Tox and Appl Pharm. 1973; 24: 239-249.
8. OShaughnessy, DJ, Losos, GJ. Peripheral and central nervous system lesions caused by triethyl- and trimethyltin salts in rats. Tox Path. 1986; 14(2).
9. Peterson, ME. Bromethalin Topical Review. Topics in Compan An Med. 2013; 28:21-23.
10. van Lier, RB, Cherry, LD. The toxicity and mechanism of action of bromethalin: a new single-feeding rodenticide. Fundam Appl Toxicol. 1988 Nov; 11(4):664-72.