Signalment:
10-year-old, female, mixed Labrador retriever, (
Canis familiaris) The dog was presented for evaluation of decreased mobility of 3 weeks duration culminating in intermittent tetraparesis. On neurological examination the dog had severe ataxia, was able to stand with support, but would knuckle in all limbs and was most weak on the right thoracic limb, which had noticeable biceps and triceps muscle atrophy. Decreased withdrawal was observed in the right thoracic limb. Conscious proprioceptive deficits were present in all four limbs. There was severe pain on cervical palpation or manipulation. With magnetic resonance neuroimaging, axial slices from cervical cord segments C1 to C3 detected a well defined dural-based mass in the right dorsal lateral aspect of the spinal canal cranial to C2, causing severe compression and displacement of the spinal cord ventrally and to the left. The mass caused focal dilatation of the subarachnoid space at C2. The mass was isointense and hyperintense on T1 and T2 weighted images respectively. There was homogeneous intense contrast enhancement of the mass. From a surgical biopsy of the mass a diagnosis of chordoid meningioma was made and the dog was euthanized at the owners request seven days later due to a poor prognosis.
Gross Description:
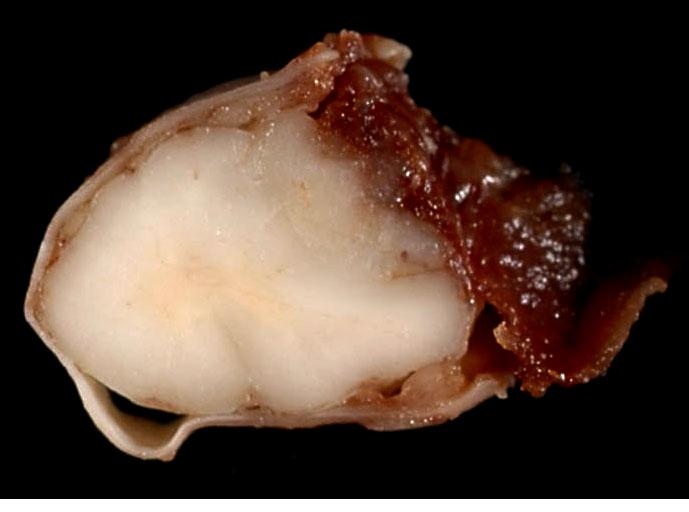
On the right dorsolateral aspect of C2, a grayish, smooth, gelatinous, partly translucent, poorly demarcated intradural mass measuring 1.5 cm x 1 cm x 0.5 cm was firmly attached to the dura mater. On transverse section the mass had indistinct borders and compressed the spinal cord to the left and ventrally (
Fig. 4-1).Â
Histopathologic Description:
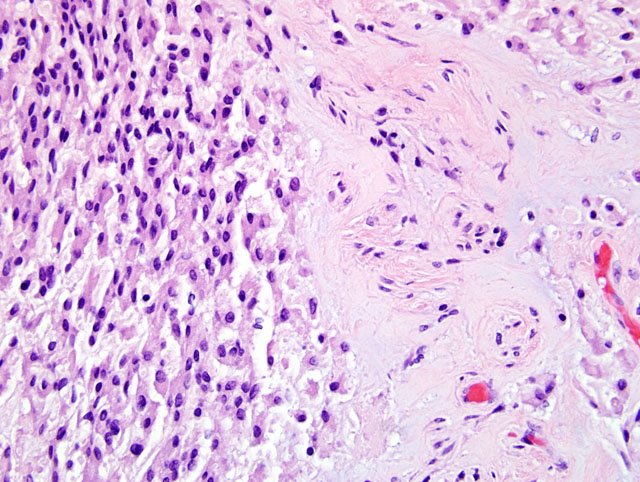
On histological exam of transverse sections of the spinal mass an intradural, extraparenchymal, well-demarcated tumor was on the right dorsal aspect of the spinal cord, causing severe displacement of the cord ventrally and to the left. The tumor was organized in a pattern of trabeculae, clusters, or columns of cells in a basophilic mucinous matrix (
Fig. 4-2). The cells were uniformly polygonal with an abundant eosinophilic cytoplasm, with a round to oval nucleus and sometimes with a prominent nucleolus and had a well-defined cytoplasmic border. Mitotic figures were rare. At the periphery of the mass were prominent blood vessels while intratumorally there were multifocal inflammatory cell infiltrates of predominant lymphocytes. One dorsal nerve root fascicle was invaded by neoplastic cells. On the lateral aspect of the cord, there was also extensive hemorrhage and fibrin deposition in addition to an amorphous basophilic foreign material consistent with surgical gelfoam associated with the previous biopsy site. The white matter of the spinal cord, compressed by and adjacent to the tumor, had multifocal sites of axon spheroids, axonal necrosis, demyelination and macrophages. The neoplastic cells were uniformly strongly immunoreactive for vimentin and focal subpopulations of cells (about 15% total) were immunoreactive for cytokeratins using a low and high molecular weight antibody cocktail (Lu5, Biocare Medical). The proliferative index, assessed by MIB1 immunoreactivity, was approximately 1%.
Morphologic Diagnosis:
Spinal Cord (Segments C2-3): Chordoid Meningioma
Condition:
Choroid meningioma
Contributor Comment:
This tumor was classified histologically as a chordoid meningioma based on location, morphology and immunohistochemistry and using criteria from the latest update of the human WHO classification of tumors of the central nervous system.(5) Although this tumor was relatively benign cytologically, in humans chordoid meningiomas are graded as atypical (WHO Grade II) based on the high incidence of post surgical recurrence.(2,5) Certainly the invasive nature of this tumor within nerve roots fascicles would support this grade. Classification of canine meningiomas using the human WHO classification into one of three histological grades (benign, atypical, anaplastic) using carefully defined and clinically tested criteria in humans may also be useful for predicting clinical behavior and outcome in dogs.(6,8)
In dogs spinal cord chordoid meningiomas have been reported with a predilection site in the cervical region as in this case; (6,8) however, in another series of canine meningiomas this type occurred in the forebrain.(1) Confusingly, these tumors have been classified for unknown reasons as myxoid meningiomas in the veterinary literature. The chordoid type is a histological variant of meningioma, defined histologically by cords or trabeculae of polygonal cells with an eosinophilic cytoplasm in an abundant basophilic mucinous matrix. Chronic inflammatory cell infiltrates may be prominent as in this case.(2,5) In humans this chordoid pattern is not an uncommon focal pattern in atypical or aggressive meningiomas, however it is rare in pure form.(2) Meningiomas in people and dogs are uniform and strongly immunoreactive for vimentin and subpopulations of cells are variably immunoreactive for pancytokeratins.(1,2,5,9) Many human meningiomas are immunoreactive for epithelial membrane antigen and this is therefore a useful diagnostic feature, but unfortunately this epitope is not expressed in the dog.Â
The differential diagnoses for chordoid meningiomas include: chordoma, myxoid chondrosarcoma, and metastatic carcinoma. Chordomas arise from the axial skeleton, have characteristic fibrous lobularity, the hallmark physaliphorous cells and are strongly uniformly immunoreactive for low-molecular-weight cytokeratin. Myxoid chondrosarcomas do not exhibit immunoreactivity for keratin. Chordoid meningiomas lack the anaplasia of metastatic carcinomas (2).Â
JPC Diagnosis:
Cervical spinal cord, meninges: Meningioma, chordoid (myxoid) with focal hemorrhage and surgical gel
Conference Comment:
Meningiomas are derived from meningothelial cells of the arachnoid membrane.(4) Meningiomas tend to be discrete, well-demarcated tumors with a broad attachment to the meninges.(4) They rarely metastasize.(7)
There are nine meningioma subtypes listed in the second series of the WHO International Histological Classification of Tumors of Domestic Animals: meningotheliomatous, fibrous, transitional, psammomatous, angiomatous, papillary, granular cell, myxoid, and anaplastic.(3) The myxoid variant is comparable to the chordoid variant as seen in humans. As one of the aims of the WHO International Histological Classification of Tumors in Domestic Animals was to follow the WHO histological classification for human tumors as closely as possible to facilitate communication between pathologists, diagnosticians and researchers, perhaps the chordoid terminology should have been used.Â
In a recent publication, canine meningiomas were divided into histologic grades according to the WHO classification used for human meningiomas. 56% of canine meningiomas were grade I, 43% were grade II and 1% were grade III, as compared to 80% grade I, 8% grade II and <3% grade III meningiomas reported in humans.(8) The biological significance of these differences is not known.Â
References:
1. Barnhart KF, Wojcieszyn J, Storts RW: Immunohistochemical staining patterns of canine meningiomas and correlation with published immunophenotypes. Vet Pathol
39:311-312, 2002
2. Burger PC et al: Surgical Pathology of the Nervous System and Its Coverings, 4th ed., pp. 59-61. Churchill Livingstone, New York, NY, 2002
3. Koestner A, Bilzer T, Fatzer R, Schulman FY, Summers BA, Van Winkle TJ: Histological classification of tumors of the nervous system of domestic animals.Â
In: World Health Organization Histological Classification of Tumors of Domestic Animals, ed. Schulman FY, Second Series, vol 5, pp. 27-29. Armed Forces Institute of Pathology, Washington, DC, 1999
4. Koestner A, Higgins RJ: Tumors of the nervous system.Â
In: Tumors in Domestic Animals, ed. Meuten DJ, 4th ed., pp. 703-706. Iowa State Press, Ames, Iowa, 2002
5. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P: The 2007 WHO classification of tumours of the central nervous system. Acta Neuropath
114(2):164-172, 2007
6. Petersen SA et al: Canine intraspinal meningiomas: imaging features, histopathologic classification, and long-term outcome in 34 dogs. J Vet Intern Med
22(4):946-53, 2008
7. Schulman FY, Ribas JL, Carpenter JL, Sisson AF, LeCouteur RA: Intracranial meningioma with pulmonary metastasis in three dogs. Vet Pathol
29:196-202, 1992
8. Sturges BK et al. Magnetic resonance imaging and histological classification of intracranial meningiomas in 112 dogs. J Vet Intern Med
22:586-595, 2008
9. Van Winkle TJ, et al: Myxoid meningiomas of the rostral cervical spinal cord and caudas fossa in four dogs. Vet Pathol
31:468-471, 1994