Signalment:
Gross Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
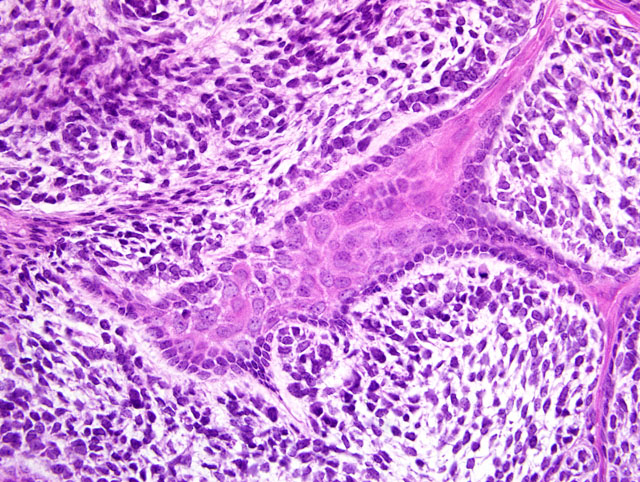
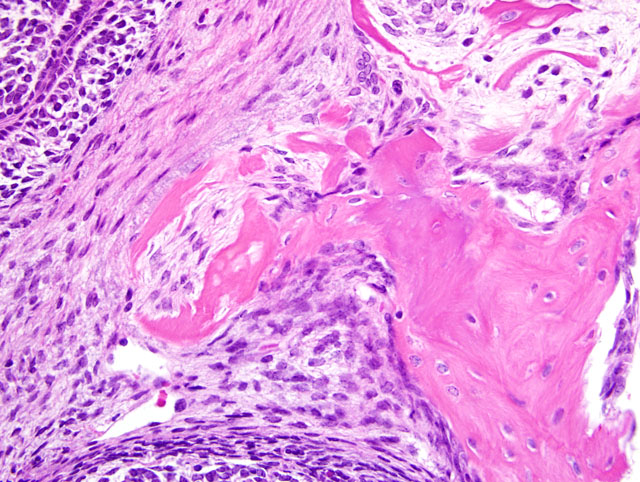
Odontogenic neoplasms are the most common spontaneous neoplasms in Tg.AC mice occurring at an incidence of approximately 13% in males and 17% in females.(2) Grossly, they occur as firm masses in the maxilla or mandible. Microscopically, 3 morphologic types occur the most frequent of which is the ameloblastic type as in the present case submission.(2,3) The second type, which appears to arise from the periodontal ligament, is the mesenchymal type which is composed of mesenchymal cells embedded in a dense eosinophilic matrix. The third morphologic type resembles odontomas and is composed largely of irregular abortive tooth structures formed by enamel, dentin and well differentiated ameloblasts and odontoblasts.
Other common neoplasms of Tg.AC transgenic mice include squamous papillomas of the forestomach, skin papillomas, alveolar/bronchiolar adenoma, salivary gland duct carcinoma and erythroleukemia seen primarily in the liver with involvement of the spleen, bone marrow and lymph nodes.
JPC Diagnosis:
Conference Comment:
All participants slides contained a pre-existing tooth at the periphery of the neoplasm. In addition, some participants slides contained a smaller tooth-like structure within the neoplasm. Considerable discussion centered on the origin of this structure, with some participants interpreting it as tooth recapitulation by neoplastic cells, while other participants favored a pre-existing structure. The classification of odontogenic tumors in transgenic mice, as summarized by the contributor, is convenient and avoids the problem of interpreting this tooth-like structure. However, participants noted that classification using the World Health Organizations (WHOs) scheme for domestic animals would hinge on interpretation of this structure. If this structure is interpreted as recapitulation by the neoplasm, the diagnosis would be ameloblastic fibro-odontoma, while the absence of this feature would make the tumor consistent with an ameloblastic fibroma using the WHO scheme.(1)
While in domestic species most odontogenic tumors have similar biological behavior (i.e. local destruction by expansion, amenable to surgical excision), there are two notable exceptions. Canine acanthomatous ameloblastoma is aggressive, locally infiltrative, and prone to recurrence; fibromatous epulis of periodontal ligament origin is benign, and a surgical cure is often achieved despite incomplete surgical margins.(1)
The high incidence of odontogenic tumors in Tg.AC transgenic mice is attributed to the expression of the ras oncogene. Odontogenic tumors appear in up to 100% of dual transgenic mice expressing both ras and myc oncogenes, and appear as early as 4 weeks of age.(3) Oncogenes are constitutively active genes that promote autonomous cell growth in cancer cells in the absence of normal growth-promoting signals. Oncogenes result from mutations in proto-oncogenes, and their products are called oncoproteins. Normal ras proteins are bound to the cytoplasmic aspect of the plasma membrane, and are activated only upon growth factor binding to plasma membrane receptors. Activated ras stimulates the mitogen-activated protein kinase cascade, resulting in signals to the nucleus for cell proliferation. Mutated ras proteins are continually activated, usually due to point mutations that impair guanosine trhiphosphate (GTP) hydrolysis, resulting in continuous stimulation of downstream proliferation signals. The mechanisms by which myc oncogene expression influences cell proliferation are less clear. Myc modulates myriad cellular activities, and is thought to be involved in carcinogenesis via some of its many targets, including ornithine decarboxylase and cyclin D2.(5)
References:
2. Mahler JF, Flagler ND, Malarkey DE, Mann PE, Haseman JK, Eastin W. Spontaneous and chemically induced proliferative lesions in Tg.AC transgenic and p53-heterozygous mice. Toxicologic Pathology 26(4):501-511, 1998
3. Mahler M, Rozell, Mahler JF, Merlino G, Devor-Hennemn D, Ward JM, Sundberg JP. In: Pathology of Genetically Engineered Mice. Eds. Ward JM, Mahler JF, Maronpot RR, Sundberg JP, Fredrickson RM, 1st edition. Iowa State University Press, Ames, IA, 2000
4. National Toxicology Program CD-Rom, Laboratory of Experimental Pathology. Lesions of Genetically Altered Mice, 2001 (http://dir.niehs.nih.gov/dirlep/genmice2/open_me.htm)
5. Stricker TP, Kumar V: Neoplasia: In: Robbins and Cotran Pathologic Basis of Disease, eds. Kumar V, Abbas AK, Fausto N, Aster JC, 8th ed., pp. 279-286. Saunders Elsevier, Philadelphia, PA, 2010