Signalment:
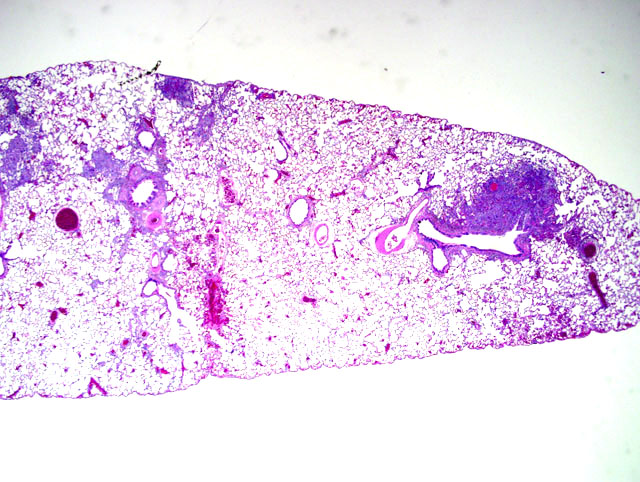
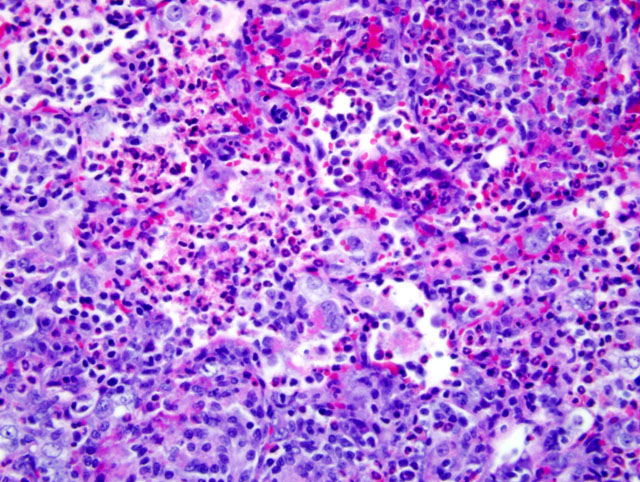
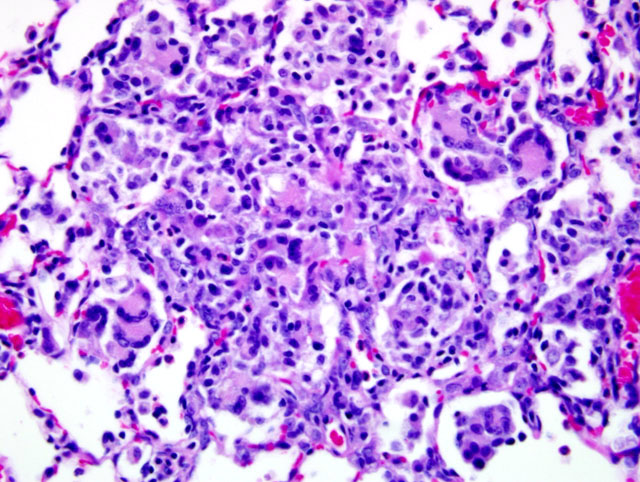
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Because of this unique immunologic response, the Brown Norway (BN) rat has served as a model for asthma in humans. BN rats also tend to develop a spontaneous eosinophilic and granulomatous pneumonia that is most frequently observed in young females. The lesion consists of epitheliod cells and numerous multinucleated giant cells admixed with an eosinophilic inflammatory infiltrate. To date the pathogenesis had not been discovered and no infectious agent has been identified.9
JPC Diagnosis:
Conference Comment:
In the Brown Norway strain, submucosal glands are larger and more numerous, particularly in the middle and lower trachea, as compared to F344, but the histochemical nature of the mucin produced by these cells is the same. The relationship between the quantitative difference in the number of submucosal glands and the Brown Norway rat's predisposition for hyperresponsiveness to allergens is still under investigation.6 It has been proposed that mucosal IgA and interleukin-8 stimulate the activity of eosinophils.2,11 Following exposure to 1% ovalbumin (OVA) solution, Brown Norway rats exhibited increased levels of Th1- (IFN-γ and IL-2) and Th2-related cytokines (IL-4 and IL-5) and chemokines (eotaxin and MCP-1) as compared to exposed F344 rats.8 Alveolar macrophages have been shown to produce significantly more nitric oxide, IL-10 and TNF when exposed to ovalbumin than similarly challenged Sprague-Dawley rats, indicating that alveolar macrophages have a role in expanding the Th2 response in allergic inflammation.1
Other strain-specific responses of the Brown Norway rat include a high susceptibility to Th2-mediated auto-immune disease such as mercury (HgCl2)-induced autoimmune glomerulonephritis. They have a vigorous Th2 immune response, producing cytokines IL-4, IL-6, and IL-10, along with antibody isotypes IgG1 and IgE on antigenic stimulation.3
References:
2. Erger RA, Casale TB: Interleukin-8 is a potent mediator of eosinophil chemotaxis through endothelium and epithelium. Am J Physiol 268:L117-L122, 1995
3. Jennings VM, Dillehay DL: Immunology. In: The Laboratory Rat, eds. Suckow MA, Weisbroth SH, Franklin CL, 2nd ed., p. 854. Elsevier Inc., New York, NY, 2006
4. Kohn DF, Clifford CB: Biology and diseases of rats. In: Laboratory Animal Medicine, eds. Fox JG, Anderson LC, Loew FM, Quimby FW, 2nd ed., p. 156. Elsevier Science, San Diego, CA, 2002
5. Michielsen CP, Leusink-Muis A, Vos JG, Bloksma N: Hexachlorobenzene-induced eosinophilic and granulomatous lung inflammation is associated with in vivo airways hyperresponsiveness in the brown Norway rat. Toxicol Appl Pharmacol 172:11-20, 2001
6. Ohtsuka R, Doi K, Itagaki S: Histological characteristics of respiratory system in Brown Norway rat. Exp Anim 46:127-133, 1997
7. Ohtsuka R, Doi K: Environmental effect on eosinophilic granulomatous pneumonia (EGP) in Brown Norway rats. J Toxicol Pathol 16:129-131, 2003
8. Ohtsuka R, Shutoh Y, Fujie H, Yamaguchi S, Takeda M, Harada T, Doi K: Changes in histology and expression of cytokines and chemokines in the rat lung following exposure to ovalbumin. Exper Toxicol Pathol 56:361-368, 2005
9. Percy DH, Barthold SW: Rat. In: Pathology of Laboratory Rodents and Rabbits, 3rd ed., pp. 125-177121. Blackwell Publishing, Ames, IA, 2007
10. Schneider T, van Velszen D, Moqbel R, Issekutz AC: Kinetics and quantitation of eosinophil and neutrophil recruitment to allergic lung inflammation in a brown Norway rat model. Am J Respir Cell Mol Biol 17:702-712, 1997
11. Shute JK, Lindley I, Peichl P, Holgate ST, Church MK, Djukanovi_ R: Mucosal IgA is an important moderator of eosinophil responses to tissue-derived chemoattractants. Int Arch Allergy Immunol 107:340-341, 1995