Signalment:
Gross Description:
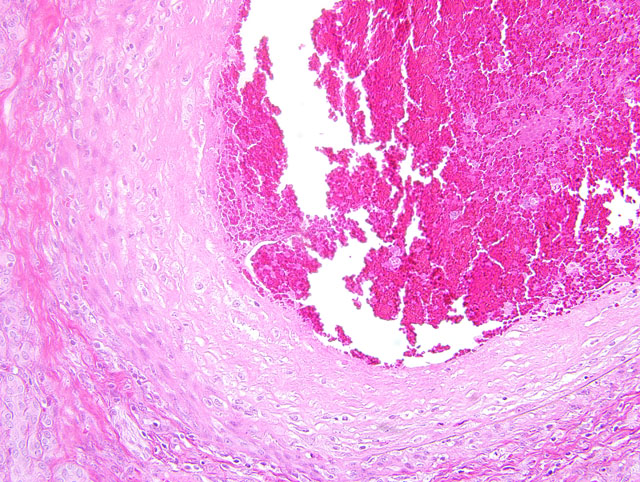
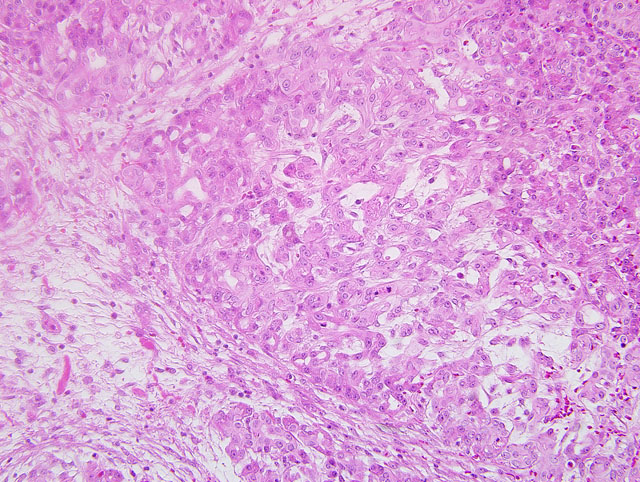
Histopathologic Description:
Morphologic Diagnosis:
1. Pancreas: Arteritis, chronic-active, proliferative, necrotizing, circumferential, transmural, severe with fibrinoid necrosis, luminal stenosis and thrombosis and multifocal pancreatic necrosis with atrophy and ectasia of acini.
2. Liver (not submitted): Arteritis, chronic-active, segmental to circumferential, transmural, severe with fibrinoid necrosis, luminal thrombosis, narrowing or obliteration of the vascular lumen, sclerosis and thickening of the arterial wall, perivascular accumulation of hemosiderin-laden macrophages, multifocal hepatic necrosis and loss of hepatic parenchyma rimmed by a lymphohistiocytic plasmacytic inflammatory infiltrate.
3. Kidney (not submitted): Arteritis, interlobular and arcuate arteries, chronic-active, circumferential, segmental, transmural, severe with fibrinoid necrosis, sclerosis and thickening of the arterial wall, hemorrhage, chronic-active, lymphoplasmacytic, tubulointerstitial nephritis, tubular dilatation, distortion, degeneration and regeneration, proteinaceous casts, neutrophilic casts, periglomerular, interstitial fibrosis and loss of nephrons.
Condition:
Contributor Comment:
Polyarteritis nodosa is a commonly occurring entity in humans. It is sporadically reported in many domestic species of animals and is characterized by necrotizing inflammation of small and medium sized arteries and most commonly involves arteries of the tongue, pancreas, heart, kidneys, mesentery, urinary bladder, testes, head and gastrointestinal tract. Impaired perfusion may result leading to hemorrhage, ulceration, infarction, and atrophy of affected tissues. The etiology is not clear; however, deposits of immune complexes have been localized in the affected arteries.(5) Microscopically, acute lesions are characterized by segmental or circumferential necrosis and fibrous thickening of the walls of arteries with varying degrees of inflammation and fibrinoid necrosis. Thrombosis of vessels may lead to infarction and hemorrhage. In chronic lesions, typically the walls may be completely fibrosed. Affected vessels may show lesions of all stages of development and both acute and chronic lesions may be present in the same vessel. (4)
Polyarteritis nodosa is commonly reported in MRL and NZB mice that are prone to autoimmune diseases.(5) In rats with experimentally induced hypertension, the occurrence of PAN is related to amount of sodium chloride in the diet.(7) Also, experimentally, it has been induced with streptozotocin, nicotinamide and several other agents.(1) In dogs, PAN is associated with rheumatoid arthritis, systemic lupus erythematosus, and beagle pain syndrome.(8) In blue foxes, it has been reported in association with Encephalitozoon cuniculi infection. Polyarteritis nodosa has been described in the brains of sows with reproductive disorders(3) and is reported to be associated with Border disease in sheep.(4) A single case of PAN has been reported in a cynomolgus monkey.(6)
The intravascular round cell population in this case was an unexpected finding. These cells were present only in areas supplied by lesioned vessels and were not present in the bone marrow. Nuclear pleomorphism, prominent and multiple nucleoli and high mitotic activity of the intravascular round cell population support malignancy; however, given the distribution it may be a response to the severe inflammation and ischemia present. The tan masses noted grossly within the spleen and liver corresponded to foci of necrosis and replacement fibrosis as a consequence of necrotizing arteritis and thrombosis. No neoplastic mass lesions were present.
JPC Diagnosis:
Conference Comment:
The contributor provides a succinct overview of PAN. Conference participants noted histopathologic similarities between the blood vessels in this case and those examined in the hearts of dogs with drug-induced vascular injury due to the administration of a phosphodiesterase inhibitor (see WSC 2009-2010, Conference 2, case III). Indeed, PAN is best regarded as a heterogenous group of arteritides, as evidenced by the assorted list of conditions that have been reported in association with the entity and summarized by the contributor. In humans, PAN is a vasculitis confined to small and medium-caliber arteries, with a predilection for branching points; arterioles, capillaries, and venules are spared, as is the pulmonary circulation. Many of the conditions that have been categorized as PAN in the veterinary literature adhere only loosely to the classic definition of PAN, and would more appropriately be referred to as systemic necrotizing vasculitides.(4)
As alluded to by the contributor, many cases of polyarteritis nodosa are thought to be caused by immune complexmediated (type III) hypersensitivity, the pathogenesis of which is divided into three phases: 1) immune complex formation, 2) immune complex deposition, and 3) immune complex-mediated inflammation and tissue injury. Phase I, i.e. immune complex formation, occurs when antibody combines with antigen in the circulation (forming circulating immune complexes) or antigen that has been previously deposited in extravascular sites (forming in situ immune complexes). As described in the recent case of membranoproliferative glomerulonephritis (WSC 2009-2010, Conference 17, case III), the inciting antigens may be of either exogenous or endogenous origin. Hepatitis B virus antigens, for example, are incriminated as the inciting cause of a subset of human cases of PAN. Phase II, i.e. immune complex deposition, remains incompletely understood; however, it appears that medium-sized immune complexes formed in slight antigen excess are the most pathogenic. The distribution of immune complex deposition in part determines the distribution of resulting lesions. For example, systemic immune complexmediated diseases result from immune complex deposition in many organs, while localized disease (e.g. glomerulonephritis, arthritis, or the Arthus reaction) results from deposition confined to specific tissues. Phase III is inflammation and tissue injury. Complement-fixing antibodies, i.e. IgG and IgM, activate complement via the classical pathway, yielding C3b and C4b as by-products; neutrophils and macrophages with receptors for these opsonins contribute to inflammation and tissue injury. The importance of this pathway in tissue injury is underscored by the observation of decreased serum levels of C3, presumably due to consumption of complement, in humans in the active phase of a systemic type III hypersensitivity reaction. Additionally, some subclasses of IgG bind to leukocyte Fc receptors, exacerbating the inflammatory response to immune complex deposition.(2)
References:
2. Kumar V, Abbas AK, Fausto N, Aster JC: Diseases of the immune system. In: Robbins and Cotran Pathologic Basis of Disease, eds. Kumar V, Abbas AK, Fausto N, Aster JC, 8th ed., pp. 201-205. Saunders Elsevier, Philadelphia, PA, 2010
3. Liu CH, Yan-Han Chiang Y-H, Chu RM, Pang VF, Lee: High incidence of polyarteritis nodosa in the brains of culled sows. J Vet Med Sci 67:125-127, 2005
4. Maxie MG, Robinson WF: Cardiovascular system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 3, pp. 72-73. Saunders Elsevier, Philadelphia, PA, 2007
5. Percy DH, Barthold SW: Pathology of Laboratory Rodents and Rabbits, 2nd ed., p. 153. Iowa State University Press, Ames, IA, 2001
6. Porter BF, Frost P, Hubbard GB: Polyarteritis nodosa in a cynomolgus macaque. Vet Pathol 40:570-573, 2003
7. Race GJ, Peschel E: Pathogensesis of polyarteritis nodosa in hypertensive rats. Circ Res 11:483-487, 1954
8. Son WC: Idiopathic canine polyarteritis in control beagle dogs from toxicity studies. J Vet Sci 5:147-150, 2004