Wednesday Slide Conference, Conference 15, Case 3
Signalment:
6-12 week old, female, hybrid, Sus scrofa domesticus, pig.
History:
Multiple weaners on the property with poor body condition and wasting, leading to death.
Gross Pathology:
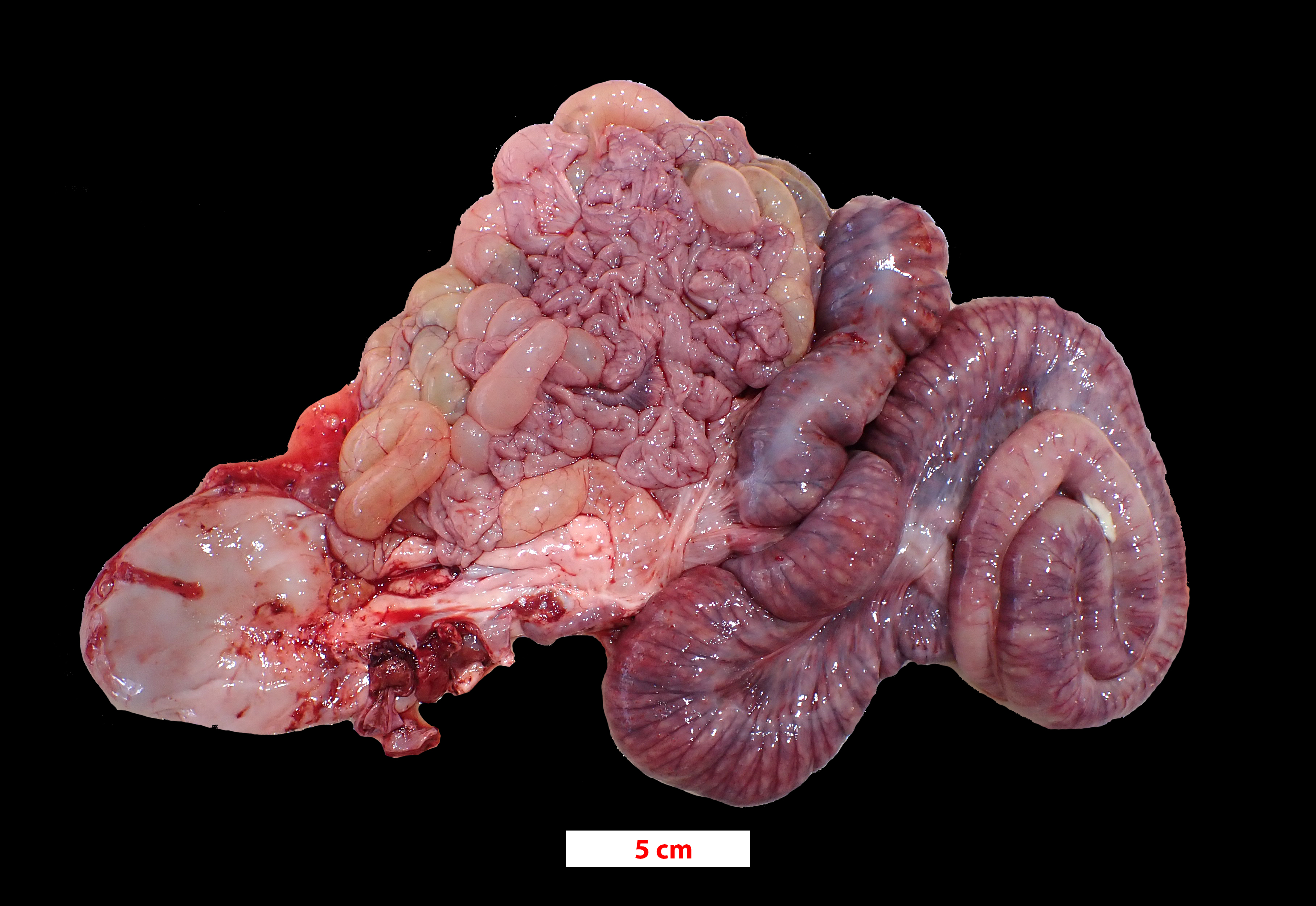
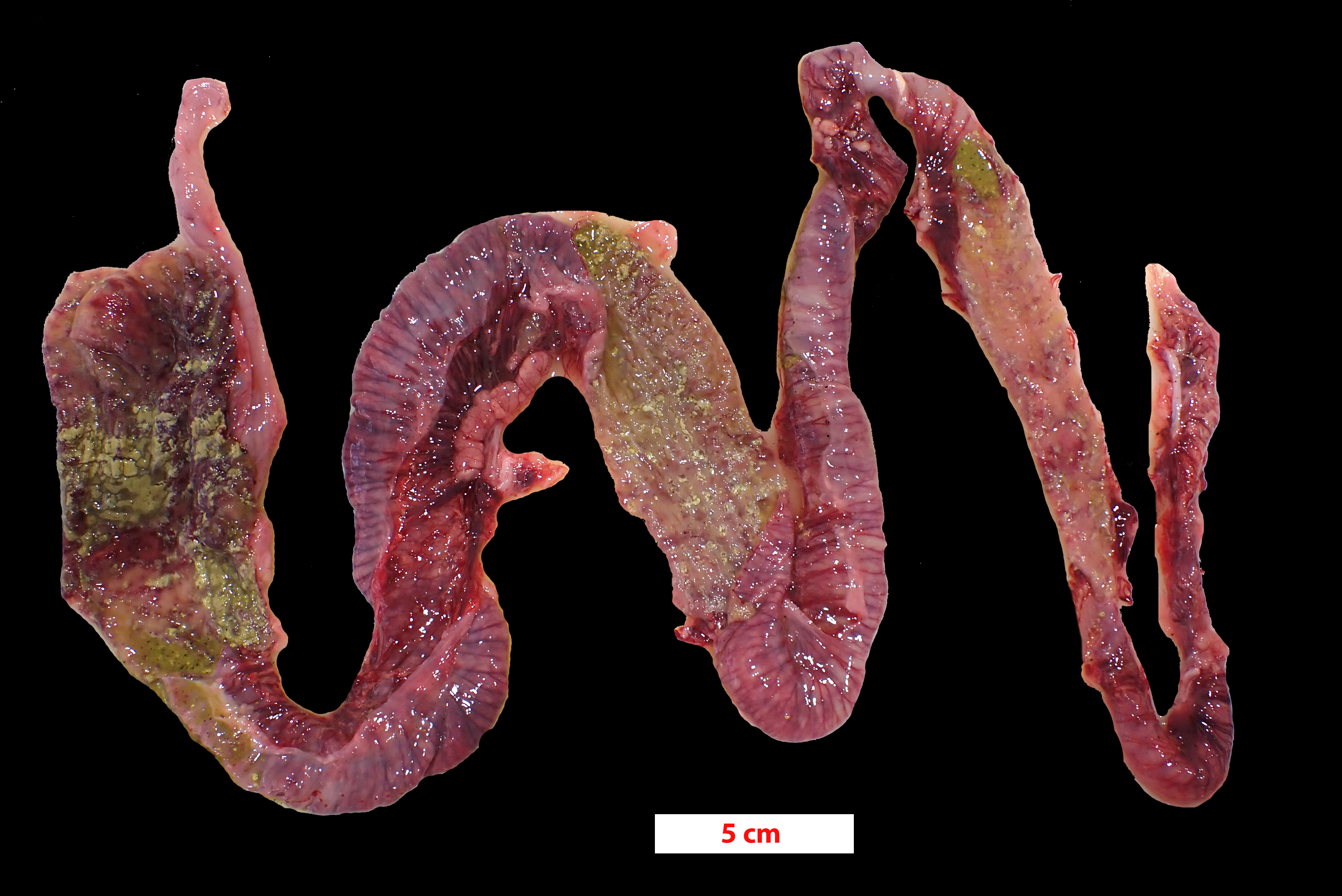
The pig was presented in poor body condition, with prominent tuber ischia (pin bones) and ribs. There was marked faecal staining around the anus. The large intestine, predominantly colon and caecum, was diffusely dilated with diffuse dark grey to red-brown discoloration of the serosa. Multifocally, the mucosa had white fibrinous lesions admixed with dark necrotic areas and areas of haemorrhage.
Laboratory Results:
Porcine circovirus type 2 real-time PCR: Positive
Salmonella enrichment culture: Positive.
Identification of Salmonella by serotyping: Salmonella enterica serovar infantis.
Brachyspira hyodysenteriae and B. pilosicoli multiplex PCR: Negative
Microscopic Description:
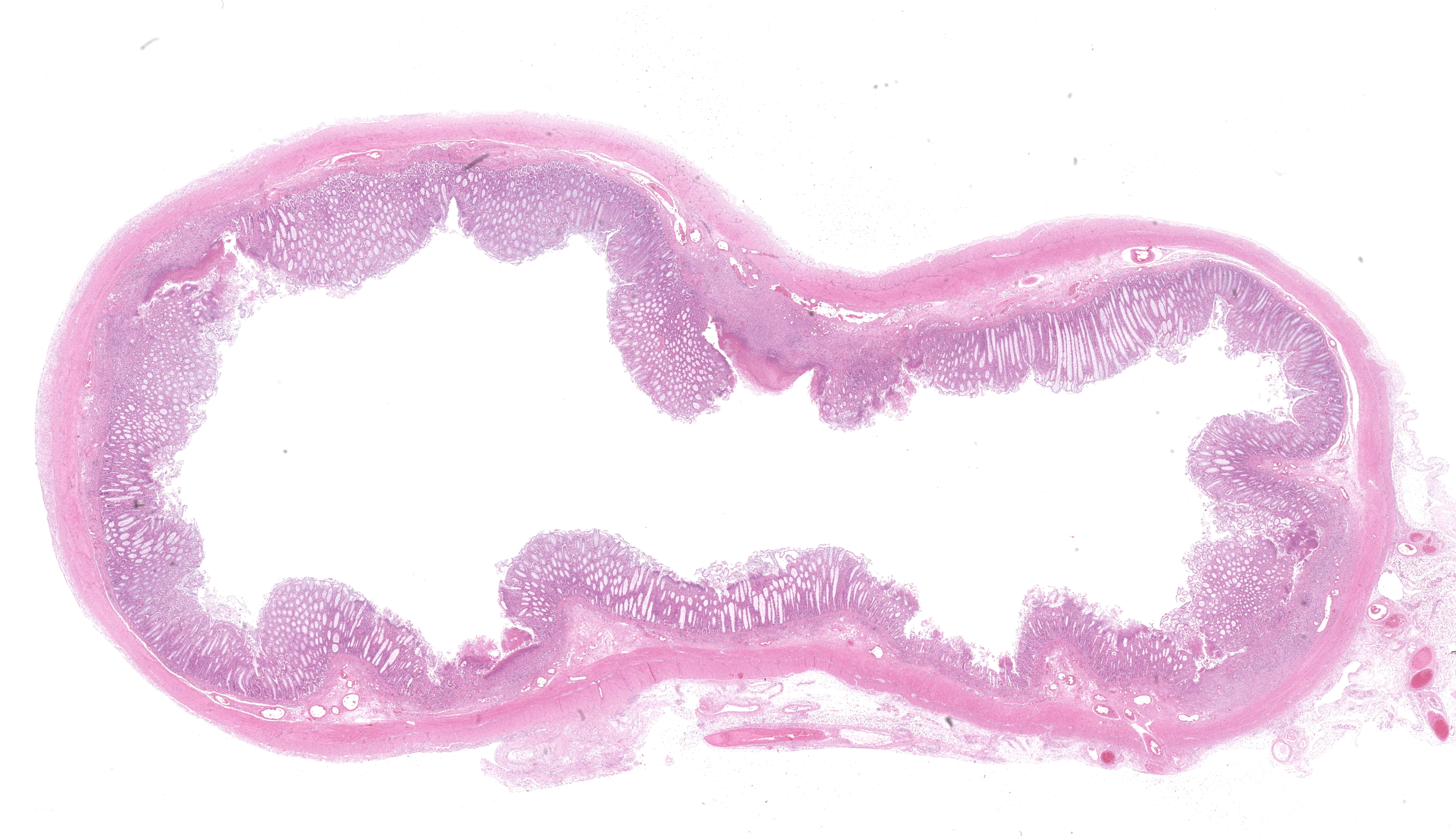
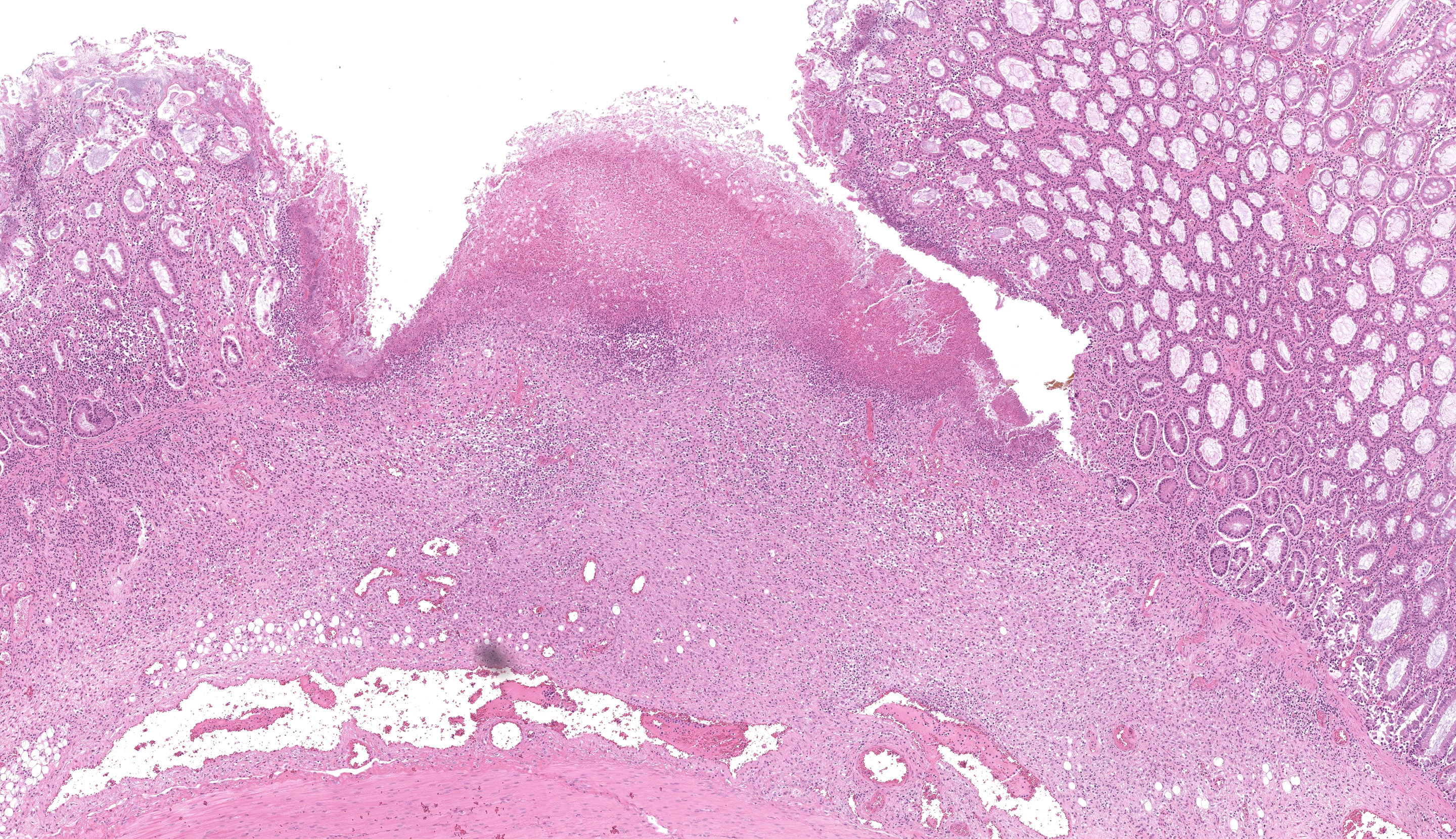
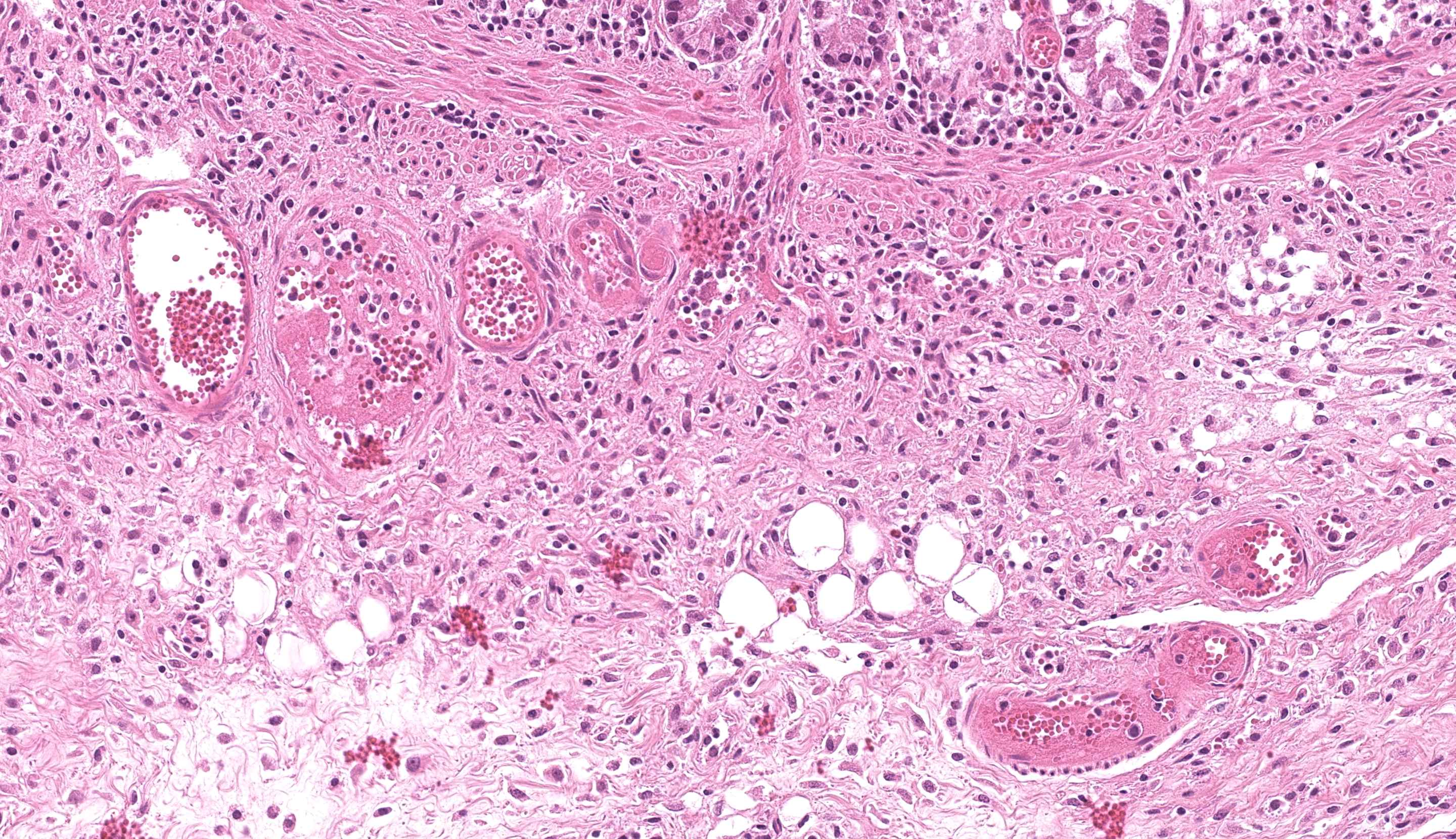
Colon or caecum (depending on the slide), H&E: Multifocally to coalescing, affecting the epithelium, lamina propria and submucosa, is marked loss of mucosa, with replacement by abundant mats of fibrillar eosinophilic material (fibrin), cellular and karyorrhectic debris, degenerate neutrophils, lymphocytes and macrophages, and colonies of basophilic bacteria with rod morphology. Small vessels in affected areas have disrupted endothelium and are often filled with thrombi. In less affected areas, crypts are markedly elongated, ectatic, with attenuated epithelium and contain amphophilic to pale basophilic fibrillar material (mucus) or filled with degenerate neutrophils and necrotic debris (crypt abscess). Multifocally, there is mild goblet cell hyperplasia. Multifocally, the lamina propria and submucosa are expanded by lymphocytes, macrophages, and plasma cells; with occasional small vessels containing thrombi. Multifocally, numerous ciliated protozoa (Balantidium coli) are free in the lumen or admixed with necrotic material and buried deep in the mucosa. The serosa is focally markedly expanded by neutrophils and fibroblastic proliferation, underlying a thin mat of fibrin, clear areas, and a florid perivascular infiltrate of lymphocytes and plasma cells.
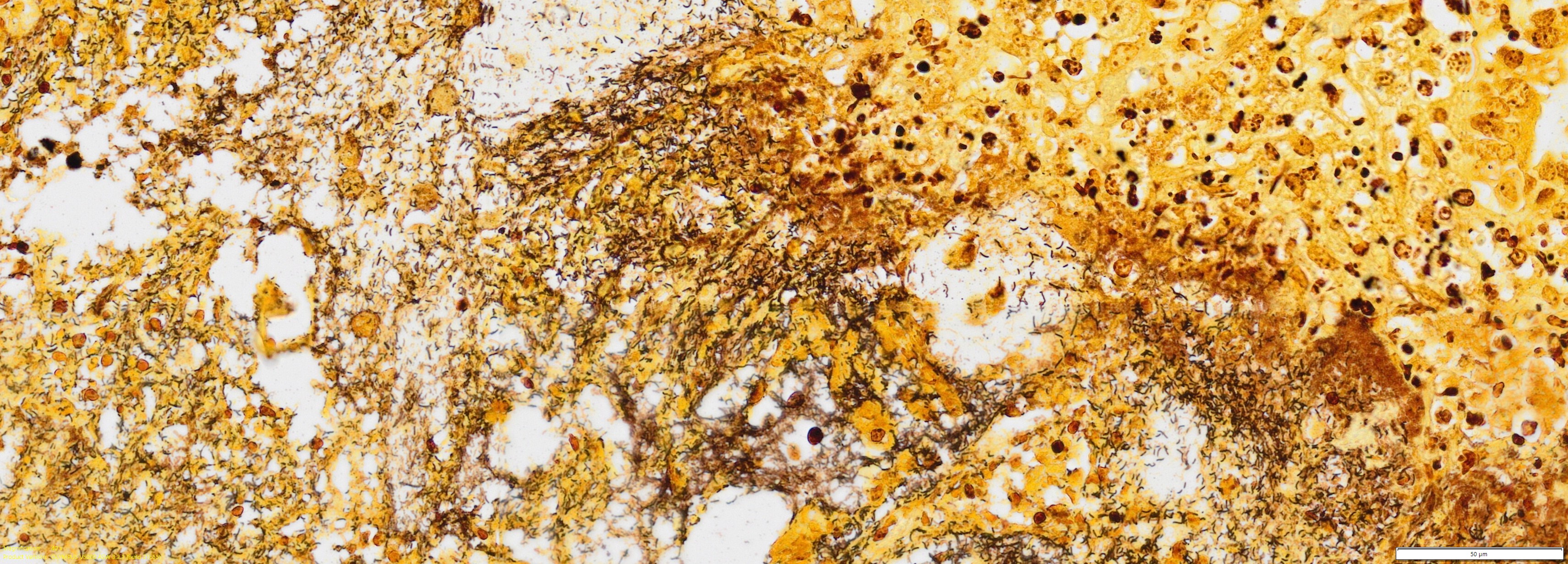
Colon or caecum (depending on the slide), Warthin-Starry: Multifocally, abundant colonies of bacteria with rod morphology are highlighted within the areas of ulceration, necrosis and inflammation, previously described in H&E.
Contributor’s Morphologic Diagnosis:
Colon or caecum: Colitis or typhlitis, fibrinonecrotizing, multifocal to coalescing, severe, with diphtheritic membranes, goblet cell hyperplasia, thrombosis, abundant intralesional rod-shaped bacteria, and numerous intralesional protozoan ciliates, hybrid breed, Sus scrofa.
Contributor’s Comment:
Salmonellosis is a common bacterial infection of mammals and birds, resulting in various syndromes such as septicaemia, enterocolitis, abortion and pneumonia. There are two recognized species of Salmonella: S. enterica and S. bongori, of which there are a vast number of serovars and serotypes.5 Additionally, there are three broad groups of Salmonella serovars to consider: those that are host adapted to humans and higher primates, those that are host adapted to certain animal species and those that are not host adapted.1
Several Salmonella enterica serovars, most commonly Typhimurium, Derby and Cholerasuis, have been identified to cause enterocolitis and septicaemia in swine;13 Cholerasuis is host adapted to swine, while Typhimurium and Derby also commonly cause disease in ruminants and poultry.
The Salmonella enterica serovar isolated in this case, Salmonella Infantis, has been identified from both clinically normal and ill pigs, as well as in the environment and carcasses of pigs at slaughterhouses.7 While this serotype is less commonly identified compared to Typhimurium, Cholerasuis, and Derby, prevalence of this serotype is increasing. In a number of studies across Canada, the United States and Brazil, Salmonella Infantis was consistently the 3rd to 4th most identified serotype in farms, slaughterhouses and in diagnostic samples at veterinary laboratories, with evidence for increasing prevalence over time.6-8,12,15 This serotype is also of public health concern due to its zoonotic potential and antibiotic resistance.4,7,9 Salmonella Infantis has been isolated in a number of food safety trials, involving both contaminated pork and poultry meat, presenting increasing risk to human health.4,10,11 Interestingly, poultry are asymptomatic carriers of Salmonella Infantis.10,14
There are no published reports of the gross or histological changes observed with Salmonella Infantis infection in swine, as far as the authors are aware. Comparatively, the gross and histological changes seen in this case are most similar to infection with Salmonella Typhimurium in swine, where diphtheritic enterocolitis is also generally confined to the large intestine and rectum, with minimal involvement of the distal ileum.13 Other bacterial agents that were considered in this case given the gross and histological findings included Brachyspira hyodysenteriae and B. pilosicoli as well as Clostridium perfringens types A and C. Both B. hyodysenteriae and pilosicoli cause fibrinonecrotic and erosive lesions limited to the colon and caecum. Diphtheritic membranes are also a common gross feature of B. pilosicoli infection. Clostridium perfringens types A and C both cause necrohaemorrhagic enterocolitis in neonatal piglets. While lesions generally involve the small intestine, severe cases also involve the large intestine.
The pathogenicity of Salmonella relies on a number of Salmonella pathogenicity islands (SPIs) and other virulence genes within the genome. The SPI-1 proteins are mainly associated with invasion of the cell, while SPI-2 proteins are involved with survival and replication within host cells; a number of virulence markers contained within SP-1 and SP-2 are present in Salmonella Infantis isolates, indicating the pathogenicity of this serotype.2 Virulence associated plasmids are also vitally important for the survival and growth of Salmonella within macrophages.2 Observable histological changes (reduction in villi length) can be present in the small intestine from one day post-infection with Salmonella Typhimurium, with severe changes such as villous atrophy and epithelial damage seen as early as two days post-infection.3
Contributing Institution:
State Veterinary Diagnostic Laboratory
Elizabeth Macarthur Agricultural Institute
Woodbridge Rd, Menangle
NSW, 2568
Australia
https://www.dpi.nsw.gov.au/about-us/services/laboratory-services/veterinary
JPC Diagnosis:
Colon: Colitis, ulcerative, subacute, multifocal, marked, with vasculitis, thrombosis, and diffuse lymphoid depletion.
JPC Comment:
We thank the contributor for sharing this slide with us as the ancillary diagnostics opened up a great conference discussion. Our Gram stain demonstrated gram-negative bacilli adjacent to ulcerated areas of the colon, consistent with the presence of Salmonella Infantis outlined above and likely the primary process in this animal though this feature was not apparent to us on H&E. Conference participants offered a number of other possible etiologies (and/or coinfections) which had varying levels of support, including Lawsonia (here lacking a marked proliferative component and the degree of hemorrhage in this case is not severe), Clostridium (Clostridioides difficile - a good differential in a newborn animal), and African or Classical Swine fever (better supported by marked hemorrhage in additional organs). We debated the relevance of Brachyspira in this case despite the PCR result as we noted many spirochetes present within colonic crypts on our silver stain as well (similar to Figure 3-7), though it is possible that this could reflect a normal amount to a slight overgrowth secondary to ulceration induced by Salmonella – the degree of mucus present on this section is not overwhelming as well. There is also a moderate number of ciliated trophozoites present within the lumen of the colon (consistent with Balantidium coli, a normal colonic commensal) which we did not assign any pathologic significance to.
One subtle feature of this case that should not be overlooked is the depletion in lymphoid cells within the colon. Simply put, there isn’t lymphoid tissue present at all in this section which underscores the need to review tissues systemically to detect severe changes. That this animal was PCR-positive for PCV-2 is a potential explanation, though there is no corroborating evidence such as cytoplasmic botryoid viral inclusions despite the vasculitis present. Salmonella is another major cause of lymphoid depletion, and often accomplishes this ‘task’ shortly after entering aggregated lymphoid nodules (GALT; Peyer’s patches) via overlying M cells. Conference participants extrapolated that the ulcers in the colon of this pig (‘button-like’) probably reflected this pathogenesis with accompanying infarction of the adjacent submucosal blood vessels.
References:
- Agbaje M, Begum RH, Oyekunle MA, Ojo OE, Adenubi OT. Evolution of Salmonella nomenclature: a critical note. Folia Microbiologica. 2011;56: 497-503.
- Almeida F, Pitondo-Silva A, Oliveira MA, Falcão JP. Molecular epidemiology and virulence markers of Salmonella Infantis isolated over 25years in São Paulo State, Brazil. Infection, Genetics and Evolution. 2013;19: 145-151.
- Bellido-Carreras N, Argüello H, Zaldívar-López S, et al. Salmonella Typhimurium Infection Along the Porcine Gastrointestinal Tract and Associated Lymphoid Tissues. Veterinary Pathology. 2019;56: 681-690.
- Borowiak M, Szabo I, Baumann B, et al. VIM-1-producing Salmonella Infantis isolated from swine and minced pork meat in Germany. Journal of Antimicrobial Chemotherapy. 2017;72: 2131-2133.
- Brenner FW, Villar RG, Angulo FJ, Tauxe R, Swaminathan B. Salmonella Nomenclature. Journal of Clinical Microbiology. 2000;38: 2465-2467.
- Clothier KA, Kinyon JM, Frana TS. Comparison of Salmonella Serovar Isolation and Antimicrobial Resistance Patterns from Porcine Samples Between 2003 and 2008. Journal of Veterinary Diagnostic Investigation. 2010;22: 578-582.
- de Quadros CL, Manto L, Mistura E, et al. Antimicrobial and Disinfectant Susceptibility of Salmonella Serotypes Isolated from Swine Slaughterhouses. Current Microbiology. 2020;77: 1035-1042.
- Farzan A, Friendship RM, Dewey CE, Muckle AC, Gray JT, Funk J. Distribution of Salmonella serovars and phage types on 80 Ontario swine farms in 2004. Canadian Journal of Veterinary Research. 2008;72: 1.
- Gal-Mor O, Valinsky L, Weinberger M, et al. Multidrug-resistant Salmonella enterica serovar Infantis, Israel. Emerg Infect Dis. 2010;16: 1754-1757.
- Hauser E, Tietze E, Helmuth R, et al. Clonal dissemination of Salmonella enterica serovar Infantis in Germany. Foodborne Pathog Dis. 2012;9: 352-360.
- Khalafalla F, Abdel-Atty N, Abdel-Wanis SA, Hanafy AS. Food poisoning microorganisms in chicken broiler meat. Glob Vet. 2015;14: 211-218.
- Possebon FS, Tiba Casas MR, Nero LA, Yamatogi RS, Araújo Jr JP, Pinto JPdAN. Prevalence, antibiotic resistance, PFGE and MLST characterization of Salmonella in swine mesenteric lymph nodes. Preventive Veterinary Medicine. 2020;179: 105024
- Uzal FA, Plattner BL, Hostetter JM. Alimentary System. Jubb, Kennedy & Palmer's Pathology of Domestic Animals: Volume 2. 2016: 1-257.e252.
- Yokoyama E, Ando N, Ohta T, et al. A novel subpopulation of Salmonella enterica serovar Infantis strains isolated from broiler chicken organs other than the gastrointestinal tract. Veterinary Microbiology. 2015;175: 312-318.
- Yuan C, Krull A, Wang C, et al. Changes in the prevalence of Salmonella serovars associated swine production and correlations of avian, bovine and swine-associated serovars with human-associated serovars in the United States (1997–2015). Zoonoses and Public Health. 2018;65: 648-661.