Signalment:
Three-year-old,
male, pigtail macaque, (
Macaca nemestrina).This animal was
inoculated intravenously with SIVmac239, 177 days prior to humane sacrifice. It
had a history of intermittent diarrhea, weight loss, mild dehydration, and
upper respiratory signs (sneezing and nasal discharge). The day before
sacrifice the animal began vocalizing and became progressively ataxic.
Gross Description:
The
animal was thin with atrophic thymus and peripheral nodes but enlarged
mesenteric and internal iliac nodes. Distal esophageal mucosa was covered with
a proliferative growth of
Candida sp. The colon had a thickened mucosa
and was dilated with fluid feces.
Histopathologic Description:
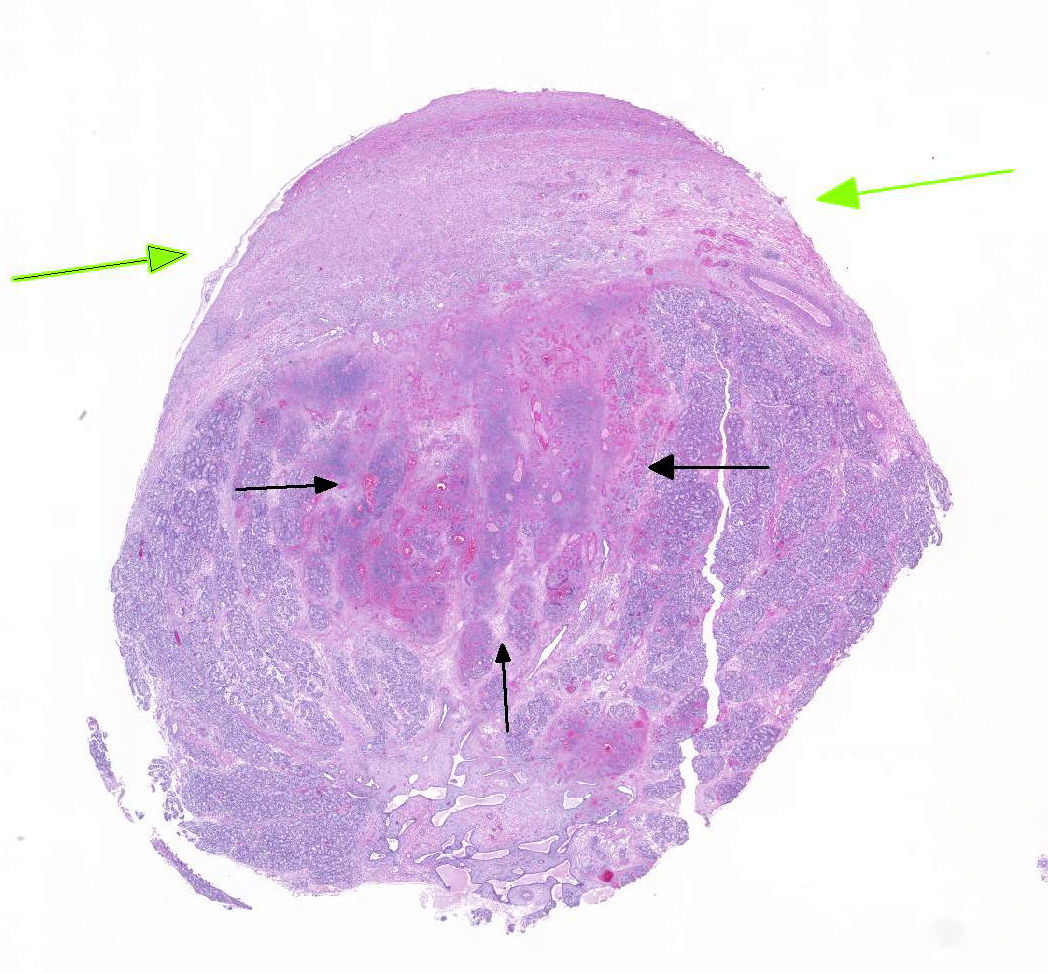
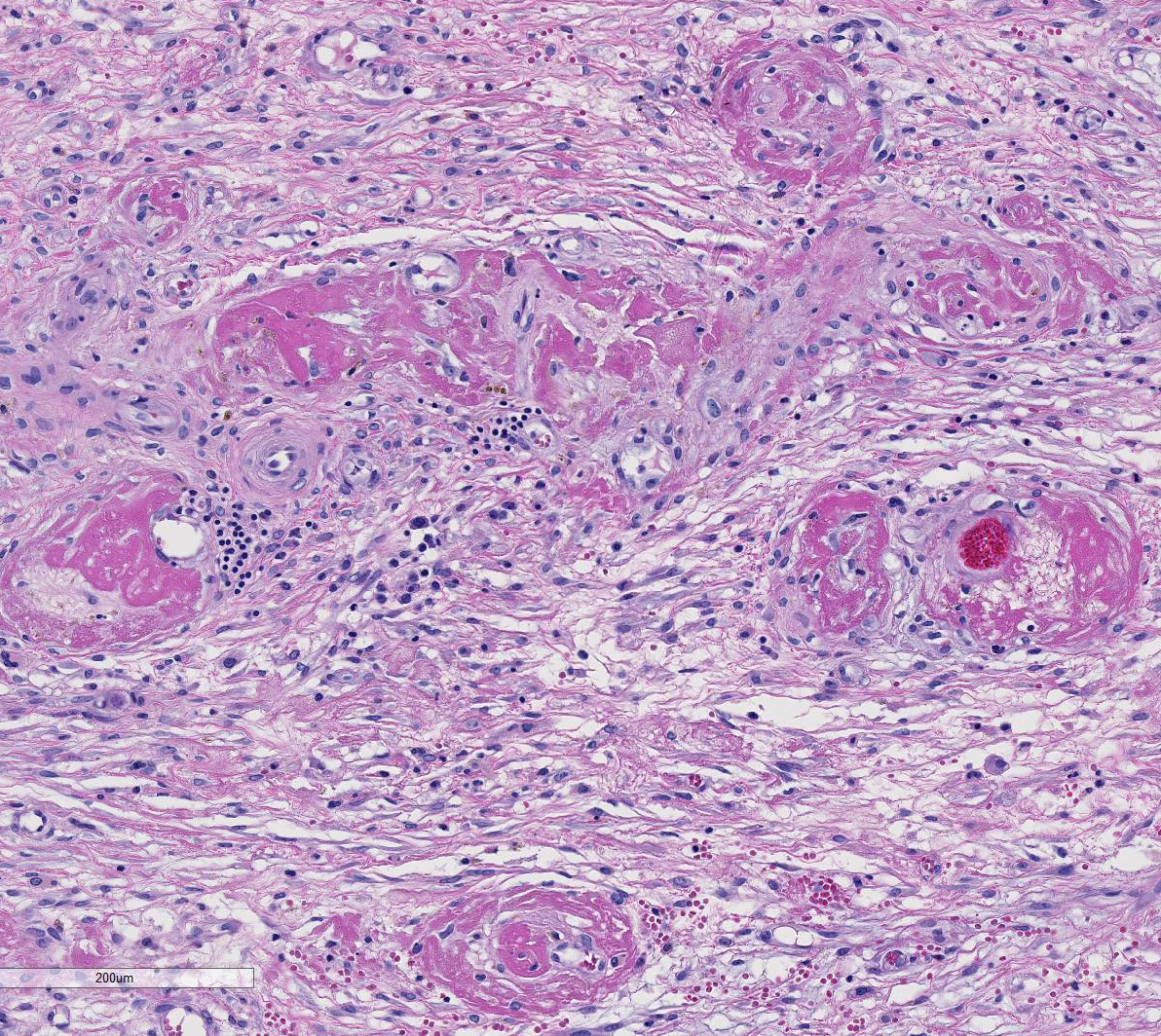
The
section of testis includes edematous rete testis with canaliculi, lobules made
up of immature seminiferous tubules with multiple zones of necrosis, and
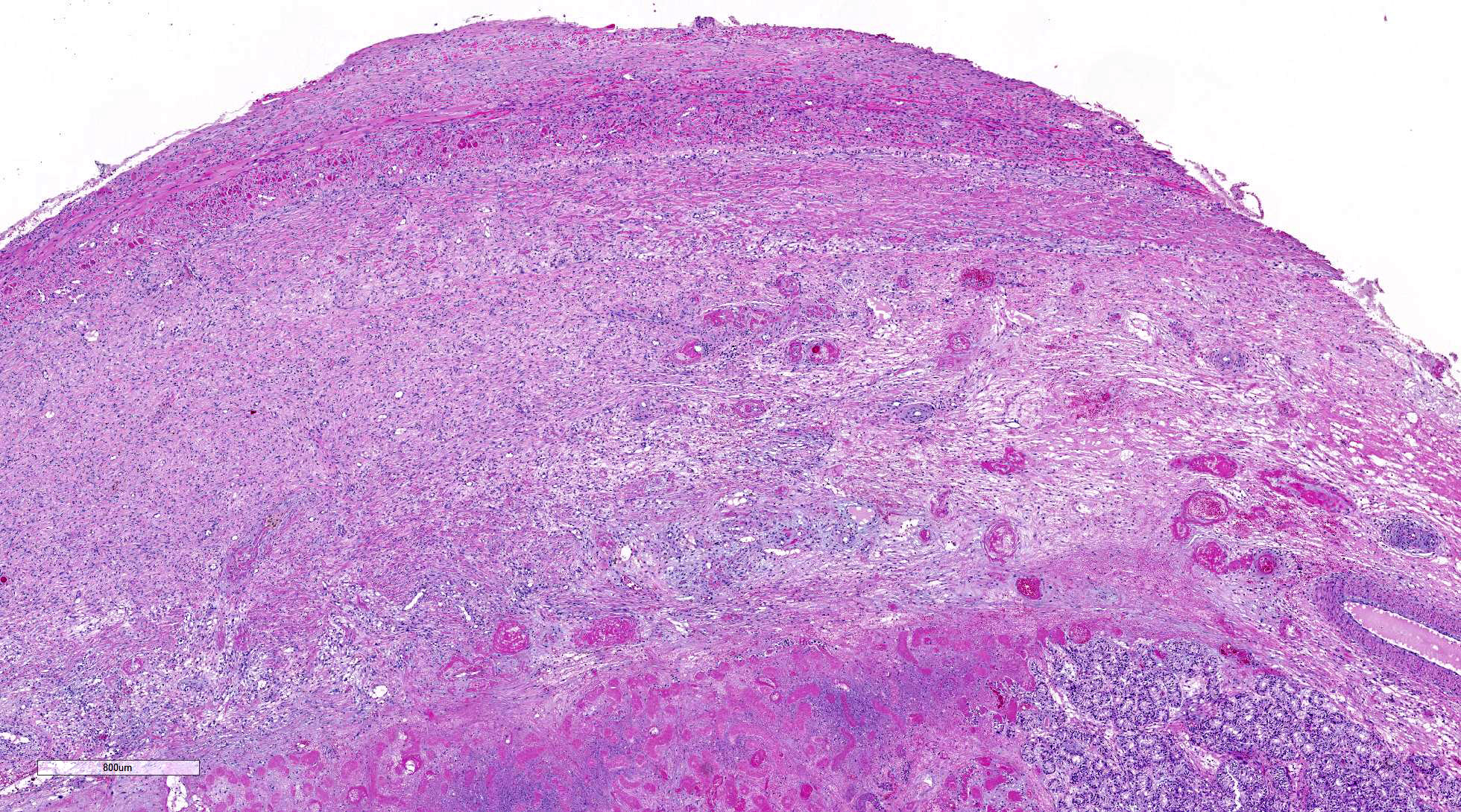
thickened, inflamed, and partially fused tunica albuginea and vaginalis.
Complete and partial lobules are destroyed by multifocal to coalescing
coagulative necrosis involving seminiferous tubules and interstitium. Segments
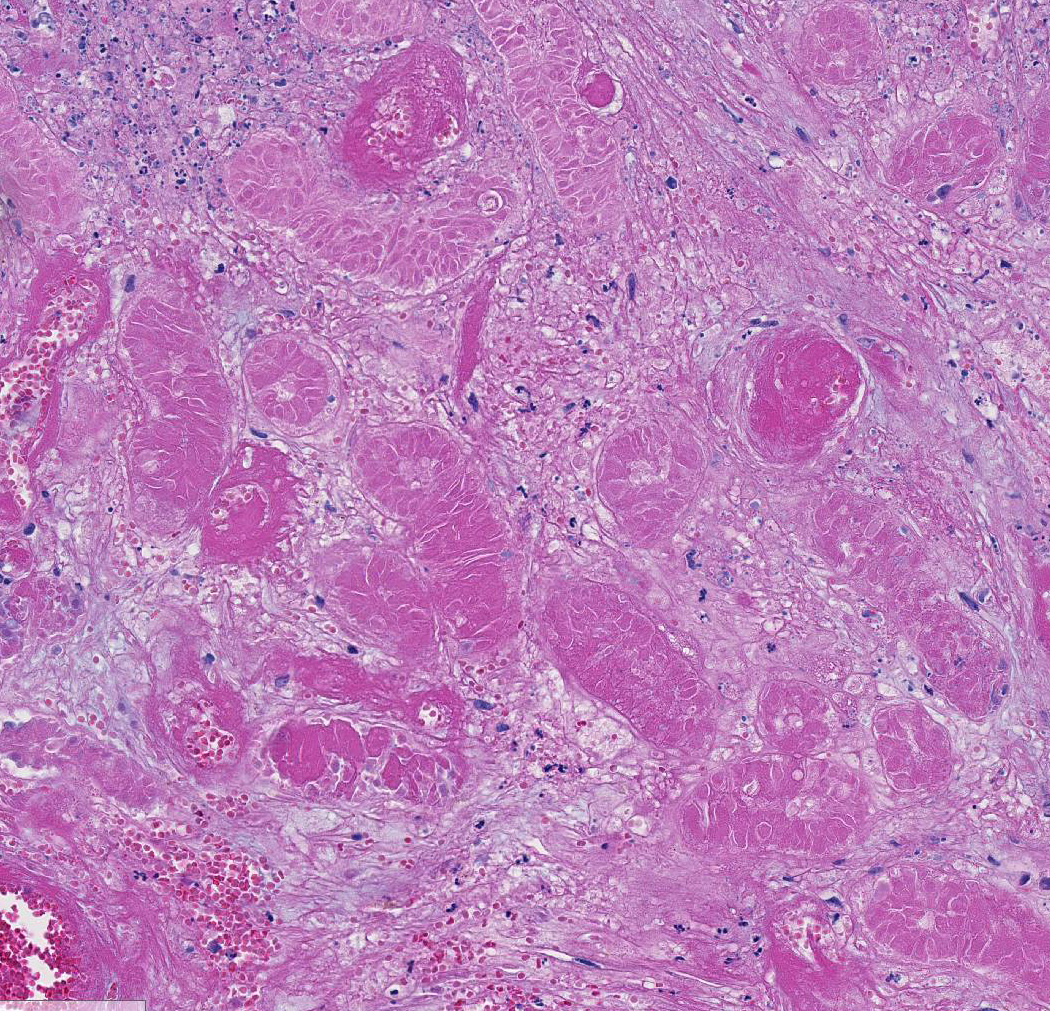
of small and medium-sized vessels are necrotic with hyalinized walls, fibrin
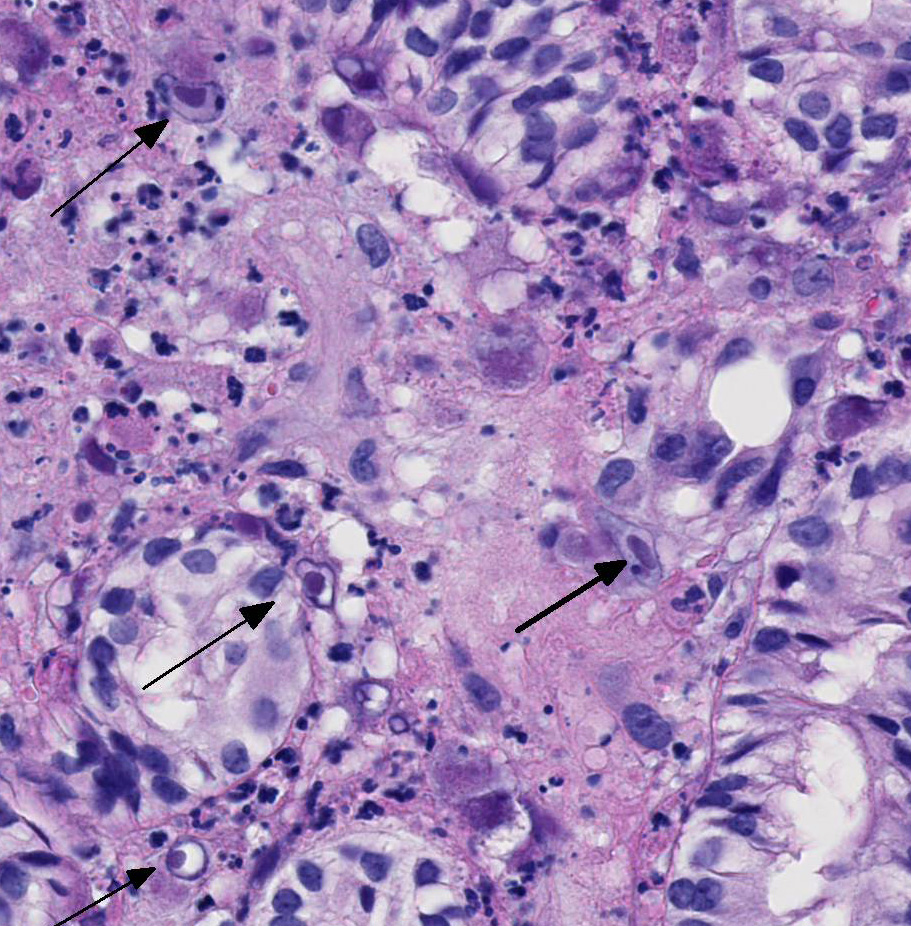
thrombi and infiltration of neutrophils. Sertoli cells within the tubules,
numerous Leydig cells, fibrocytes and myoid cells adjacent to the basal lamina
of the tubules, and fibroblasts and endothelium lining interstitial vessels
contain large round, oval, tear-drop and occasionally lobulated and multiple
eosinophilic intranuclear inclusions usually surrounded by a clear halo. Some
enlarged cells have additional amorphous granular eosinophilic aggregates
within the cytoplasm. Interstitial stroma of the mediastinum, lobules, and both
tunics is severely edematous with fibrin deposition, microhemorrhages,
microthrombi, and predominantly neutrophilic infiltration, especially around small
vessels. Neutrophils are necrotic, fragmented and mixed with small numbers of
eosinophils.
Morphologic Diagnosis:
Acute orchitis with multifocal to coalescing,
coagulative and fibrinoid necrosis, intra-vascular fibrin thrombi and intranuclear
inclusions, Cytomegalovirus, Testis
Lab Results:
Moraxella
sp
.
and hemolytic staphylococcus were cultured from the nasal cavity. Balantidium
coli and Trichuris trichiura were identified by fecal flotation.
Cytomegalovirus was identified by PCR in paraffin sections of testis.
Condition:
Necrotizing periorchitis/Rhesus cytomegalovirus
Contributor Comment:
Cytomegalovirus (CMV) is a
betaherpesvirus comparable in sequence and pathogenesis to species-specific CMV
of monkeys, humans, and other animals. CMV of Rhesus and Japanese macaques, African
green monkeys (AGM), and chimps have distinct restriction fragment profiles.1,6
Uncomplicated infections rarely cause disease, even in infants, usually become
latent, and may reactivate later in life due to the immunosuppression of viral
infection, cancer treatment, or organ transplantation. In breeding colonies,
50% of infants become seropositive by six months and nearly 100% by one year.3
Transmission via breast milk, saliva, and possibly urine2 seem
likely routes of transmission; transplacental infection is possible but rare2;
trans-plantation of infected organs is a documented risk.12
The incidence of
CMV disease in monkeys with SAIDS is variable but can be as high as 30-50% of
seropositive animals.12,15 Tissues displaying cytomegalic cells
containing intranuclear inclusions include central and peripheral nervous
system, lung, lymph nodes, liver, GI tract, testis, and arteries4.
CMV disease in SIV-infected monkeys can be
predicted by prolonged detection of CMV DNA in plasma and a decrease in anti-CMV
titer and avidity14. Profound depletion of CD8 T cells (once thought
linked9) is less important than the expanded target cell pool of
activated CD4 cells.4
Genomes for
rhesus and chimp CMV are sequenced and partial sequences for AGM and baboon CMV
are reported.6,7 There is a strong conservation of coding content
between human and simian CMV, with even closer homologies among CMV of closely
related primate hosts. While estimates of open reading frames in rhesus CMV
vary from 230-258 genes, it is clear that evolution has produced extra coding
capacity in rhesus compared to human and chimp CMV.3 Sequence
homology demonstrates codes for proteins critical for neutrophil activation by
CXC chemokines, TNF receptor, B-chemokine receptor, and IL-10 in rhesus CMV. In
human fibroblasts, CMV can change levels of more than 250 cellular genes
including cyclooxygenase 2 (COX-2)17, which converts
arachidonic acid (AA) to prostaglandin endoperoxide H. Rhesus CMV
does not increase cellular COX-2 but produces a homologue protein6
that could be used
to modulate the host inflammatory process.
CMV establishes
life-long infection in immunocompetent hosts in sites of latency and persistent
infection. During latency, infected cells demonstrate limited viral gene
expression, while in persistently infected cells; virions are continuously
produced with minimal cytopathic effect. Endothelial cells, myeloid cells
(particularly CD14+ monocytes) and possibly smooth muscle cells in large
arteries are the likely sites of infection and latency.9 CMV
infection of endothelial cells increases expression of cell adhesion molecules
(ICAM-1) which interacts with monocytes and could provide a means for
distribution. Infected cells induce a vigorous immune response releasing
pro-inflammatory cytokines (like gamma IFN and TNF-alpha) that play a role in
reactivation15. Monocyte differentiation driven by con-A-stimulated
T-cells has been shown to reactivate non-lytic infection in monocyte-derived
macrophages as well as other myeloid precursors7. The precise
combination of cells and mediators may vary depending on the system but
inflammatory cytokines, chemokines, and even some anti-inflammatory cytokines
like IL-10
play a role in reactivation. The ability of CMV to bind to Fc-domains of
neutralizing antibody and use it to infect naïve cells13 enhances
viral persistence. In one study all SIV-infected rhesus monkeys were latently
infected with CMV, seven of eleven had productive infections demonstrated by
immunohistochemistry in the gut, liver, lungs, and testicles, and two of these
seven had typical inflammatory lesions.11
Our
case demonstrates reactivation of CMV in the testis. Large numbers of classic
owls-eye cells are noted in the endothelium and interstitial cells extending
Baskins earlier observations.5 Evidence of vascular thrombosis
could have contributed to the extensive necrosis observed in this tissue.
JPC Diagnosis:
Testis:
Coagulative necrosis (infarct), focally extensive, with vascular thrombosis,
fibrinoid necrosis, chronic periorchitis with adhesions, and intranuclear viral
inclusions, pigtail macaque, Macaca nemestrina.
Conference Comment:
Rhesus cytomegalovirus (CMV), also known as macacine herpesvirus-3, is the most
common opportunistic pathogen in SIV-infected rhesus macaques with a
seroprevalence approaching 100% within the first year of life. Other nonhuman
primates with host-adapted CMVs include chimpanzees, African green monkeys,
sooty mangabeys, and owl monkeys.2,3,4,6
These highly host-specific dsDNA viruses of the subfamily betaherpesvirinae
have tropism for multiple organs producing interstitial pneumonia,
gastroenteritis, poly-radiculoneuritis, encephalitis, and lymphadenitis, in
addition to orchitis and periorchitis present in this case.2 Lesions
may also be in the liver, spleen, salivary gland, lymph node, and kidney. Both
human and rhesus CMV are unique in that they encode a CXC chemokine,
interleukin 8 (IL-8), which induces neutrophil chemotaxis, a prominent feature
in this case.2 This tissue section has marked karyomegaly and
cytomegaly with prominent magenta intranuclear inclusions, typical for CMV.
Necrotizing and proliferative vasculitis has also been reported in affected
tissues.2,3 In this case, there is fibrinoid vascular necrosis with
thrombosis, which likely caused a focally extensive area of coagulative
necrosis (infarct) in this testis.
Additionally,
a recent report in Veterinary Pathology2 reported peripheral neuropathy in the facial nerve associated
with systemic CMV infection in a group of SIV-positive rhesus macaques.
Interestingly, the pathogenesis of the nerve damage is likely due to the
bystander effect secondary to CMV-induced inflammation rather than direct viral
infection of Schwann cells.2 Readers are encouraged to review for a great example of
CMV-induced radiculitis within lumbar spinal roots of a rhesus macaque.
Conference
participants discussed other cytomegaloviruses of veterinary importance. Guinea
pig cytomegalovirus, also known as Cavid herpesvirus 1, is a common incidental
finding in immunocompetent guinea pigs. Similar to CMV of other species, the salivary
glands are the primary target tissue in the guinea pig.15
Additionally, suid herpesvirus 2 is a CMV that affects pigs causing inclusion
body rhinitis in suckling pigs and severe generalized disease in neonates
(>3 weeks old).18 Hamster, mice, and rats also have their own
host adapted CMVs typically affecting the salivary and lacrimal glands.15
References:
1. Alcendor
DJ, Barry PA, Pratt-Lowe E, Luciw PA. Analysis of the rhesus
cytomegalovirus intermediate-early gene promoter. Virology.
1993;194:815-821.
2. Assaf BT, Knight HL, Miller
AD. Rhesus cytomegalovirus (Macacine herpesvirus-3) associated
facial neuritis in a simian immunodeficiency virus infected rhesus
macaques. Vet Pathol. 2015; 52(1):217-223.
3. Barry PA and Chang WL. Primate
betaherpesviruses. In: Human Herpesviruses: Biology, Therapy, and
Immunoprophylaxis. Cambridge: Cambridge University Press; 2007.
4. Barry AP, Silvestri G, Safrit JT et
al. Depletion of CD8+ cells in sooty mangabey monkeys naturally infected
with simian immuno-deficiency virus reveals limited role for immune
control of virus replicated in a natural host species. J Immunol.
2007; 178(12):8002-8012.
5. Baskin GB. Disseminated cyto-megalovirus
infection in immuno-deficient rhesus monkeys. Am J Path. 1987;
29(2):345-352.
6. Davison AJ, Dolan A, Akter P et al.
The human cytomegalovirus genome revisited: comparison with the chimpanzee
cytomegalovirus genome. J Gen Virol. 2003; 84(1):17-28.
7. Hansen SG, Strelow LI, Franchi DC
et al. complete sequence and genomic analysis of rhesus cytomegalovirus. J
Virol. 2003; 77:6620-6636.
8. Ibanez CE, Schrier R, Ghazal P et
al. A human cytomegalovirus productively infects primary differentiated
macrophages. J Virol. 1991; 65:6581-6588.
9. Jarvis MA, Nelson JA. Molecular
basis of persistence and latency. In: Human Herpesvirus Biology,
Therapy, and Immunoprophylaxis. Cambridge University Press, 2007.
10. Kaur A, Daniel MD, Hempel D et al.
Cytotoxic T-lymphocyte responses to cytomegalovirus in normal and simian
immunodeficiency virus-infected rhesus macaques. J Virol. 1996;
70(11):7725-7733.
11. Kuhn EM, Stolte N, Matz-Rensing K
et al. Immunohistochemical studies of productive rhesus cyto-megalovirus
infection in rhesus monkeys (Macaca mulatta) infected with simian
immunodeficiency virus. Vet Pathol. 1999; 36(1):51-56.
12. Lee So, Razonable RR. Current
concepts on cytomegalovirus infection after liver transplantation. World
J Hepatol. 2010; 2(9):325-326.
13. Manley K, Anderson J, Yang F, et
al. Human cytomegalovirus escapes a naturally occurring neutralizing
antibody by incorporating it into assembling virions. Cell Host Microbe.
2011; 10:197-209.
14. Osborn KG, Prahalada S, Lowenstine
LJ et al.The pathology of an epizootic of acquired immuno-deficiency in
rhesus macaques. Am J Pathol. 1984; 114:94-103.
15. Percy DH,
Barthold SW. Pathology of Laboratory Rodents and Rabbits, 4th
ed. Ames, IA: Blackwell Publishing; 2016:15,122,175,219.
16. Sequar G, Britt WJ, Lakeman FD et
al. Experimental coinfection of rhesus macaques with rhesus
cytomegalovirus and simian immunodeficiency virus: Pathogenesis. J
Virol. 2002; 76(15):7661-7671.
17. Waldman WJ, Knight DA,
Cytokine-mediated induction of endothelial adhesion molecule and histo-compatibility
leukocyte antigen expression by cytomegalovirus-activated T cells. J
Inf Dis. 1995; 171:263-272.
18. Yoon KJ, Edington N. Porcine
cytomegalovirus. In: Straw BE, et al, eds. Diseases of Swine. 9th
ed. Ames, IA:Blackwell Publishing; 2006:323-329.
19, Zhu H, Cong JP, Mamtora G et al.
Cellular gene expression altered by human cytomegalovirus: global
monitoring with oligonucleotide arrays. Proc Natl Acad Sci 1998;
95(24):14470-14475.