Wednesday Slide Conference, Conference 12, Case 4
Signalment:
4-year-old, female, Nevisian Donkey, Equus asinus, equine.
History:
This animal initially presented with a 1-month history of chronic diarrhea and hyporexia. A fecal analysis returned a high strongyle count and the animal was subsequently treated for parasites but, there was minimal clinical improvement. An abdominal ultrasound revealed significant peritoneal effusion, an abnormal appearance to the hepatic parenchyma, and an ill-defined mass caudal to the stomach that could not be distinguished as separate from the liver. Hematology, biochemistry, and abdominal fluid analysis was performed - supporting a diagnosis of hepatic disease (see section on laboratory results). Subsequent therapy involved treatment with gentamicin and ceftiofur sodium, which was eventually discontinued when peritoneal fluid analysis was inconsistent with a peritonitis. Heparin therapy was also instituted to treat presumptive hepatic lipidosis. The donkey was found collapsed in sternal recumbency three days later and was euthanized on humane grounds.
Gross Pathology:
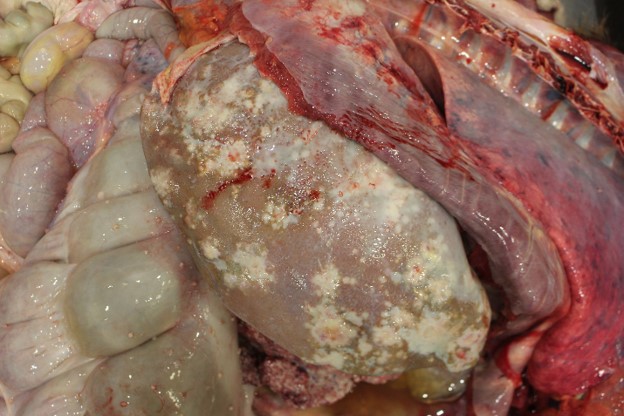
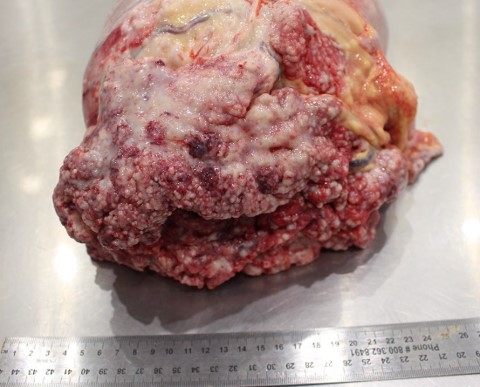
The abdominal cavity contains approximately 9.5 L of light yellow, translucent, low-viscosity fluid. Multiple (~5 mm) pale, firm raised, nodules are present throughout the peritoneum, mesentery, serosal surfaces of the intestines and on the diaphragmatic surface of the spleen. The majority of the liver contains multifocal to coalescing, firm, white-to-tan, poorly demarcated nodules that measure 0.5-2.5 cm and occasionally have central depressions that are yellow and friable (necrosis). The remainder of the liver is pale yellow, friable, and overall, the liver is enlarged with rounded edges.
There is a large (approximately 20 cm x 10 x 6 cm) multilobulated tan mass in the region of the pancreas along the greater curvature of the stomach. Intra-abdominal lymph nodes are diffusely enlarged and prominent, with multiple nodes having multifocal to coalescing white-yellow lesions expanding the parenchyma with friable, yellow centers (necrosis).
The lungs are diffusely wet and rubbery and have mild rib impressions. Similar nodular lesions to that seen in the abdominal cavity are present in the lungs.
Laboratory Results:
Initial hematology:
Hyperproteinemia (9.0, RI:6.0-8.5g/dL), anemia (PCV 26, RI: 32-48), lymphopenia (0.5, RI: 1.5-5.0 x103/μL), monocytosis (0.9, RI: 0.0-0.6 1.5-5.0 x103/μL)
Abdominal fluid analysis:
Total protein 2.4 g/dL
Nucleated cell count: 0.15 x103/μL
Interpretation: Pure transudate
Abdominal fluid culture: aerobic and anaerobic culture yielded no growth.
Biochemistry on the day of euthanasia:
|
ALP |
2261 |
50.0-170 U/L |
|
ALT |
53 |
5.0-20.0 U/L |
|
GGT |
2125 |
5.0-24.0 U/L |
|
Bile Acids |
105 |
0.0-25.0 μmol/L |
|
TBIL |
0.8 |
0.5-2.4 mg/dL |
|
ALB |
3.6 |
2.2-3.7 g/dL |
|
BUN |
9 |
7.0-25.0 mg/dL |
|
CHOL |
155 |
50.0-140.0 mg/dL |
|
TRIGLYCERIDES |
>375 |
11-68 mg/dL |
Fungal culture of the mass yielded no growth.
Aerobic and anaerobic bacterial culture of the mass yielded rare growth of mixed bacteria. Negative for Nocardia sp., Actinomyces sp. or Corynebacterium sp. No anaerobes were isolated.
Microscopic Description:
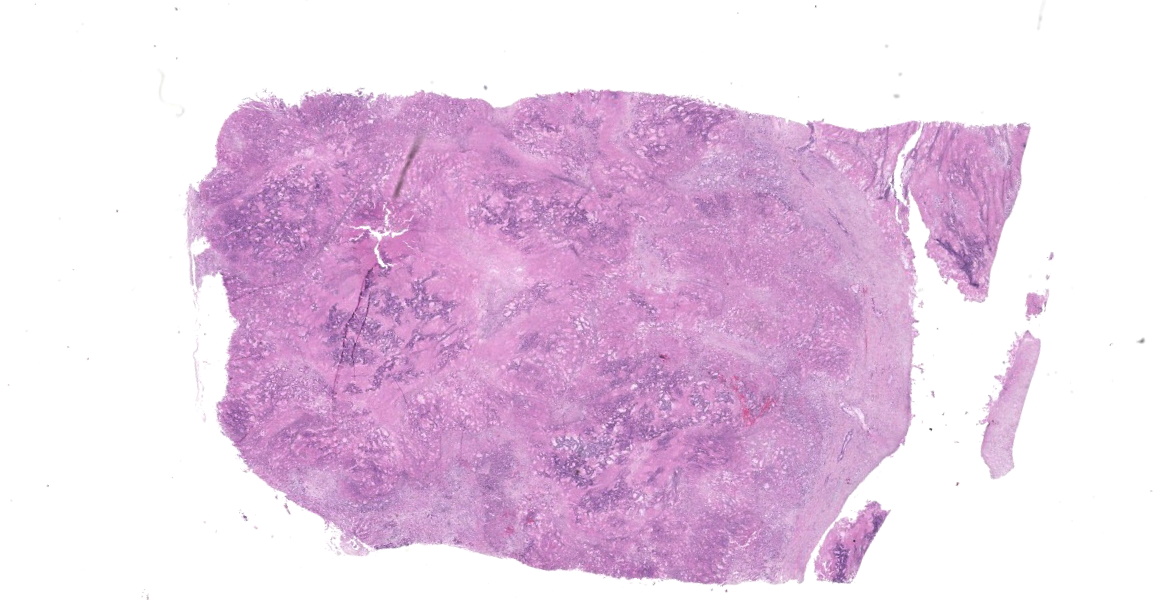
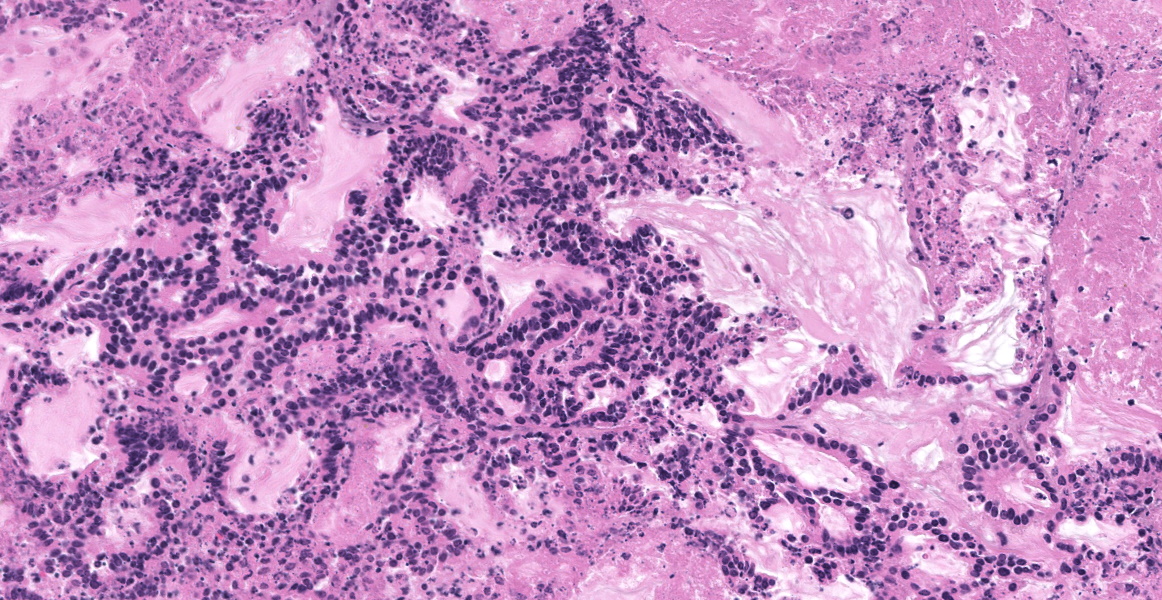
Liver: Effacing and replacing over 90% of the examined sections is a poorly demarcated, highly infiltrative, nonencapsulated neoplasm composed of islands of neoplastic epithelial cells forming cords, islands and ducts supported by moderate-to-abundant fibrocollagenous stroma. Neoplastic cells lining ductal structures are predominately columnar with a distinct brush border, variable amounts of pale eosinophilic cytoplasm, and a basilar nucleus with coarse chromatin and up to two small nucleoli. In contrast, neoplastic cells forming cords and islands are often polygonal with larger nuclei closely resembling hepatocytes. Anisocytosis and anisokaryosis are moderate and mitotic figures are rare. Rarely (and not in all sections), seemingly well-differentiated neoplastic hepatocytes appear to transition into neoplastic ductal structures. Within the neoplasm are multiple foci of coagulative necrosis and ducts are frequently filled with sloughed epithelial cells, cellular debris, and eosinophilic secretory material. Thick bands of fibrovascular tissue (scirrhous response) surround and expand the proliferating neoplastic cells and are infiltrated by low numbers of lymphocytes and plasma cells. The remaining, non-neoplastic hepatocytes have shrunken, pale eosinophilic cytoplasm and the hepatic capsule is thickened by fibrous connective tissue and lined by plump mesothelial cells.
Within sections of the pancreas, spleen, lung, and lymph node (not submitted) is a neoplastic process similar to that of the liver.
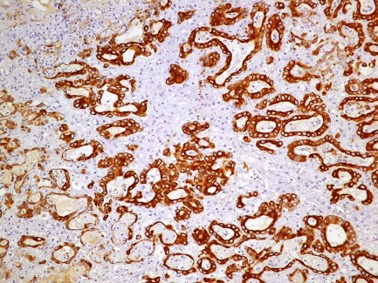
Immunohistochemistry (IHC): Sections of the mass adjacent to the pancreas and within the liver were labeled for Cytokeratin-19, and Hepar-1. The neoplastic cells forming ductal structures labeled positive with CK19 and mostly negative for Hepar-1. However, in transition zones between neoplastic cells forming cords and resembling hepatocytes and neoplastic ducts (presumably biliary) individual cells and clusters of cells in neoplastic ducts also labeled positive with Hepar-1.
Contributor’s Morphologic Diagnosis:
Liver: Carcinoma (suspect cholangiolocarcinoma)
Contributor’s Comment:
This case proved particularly challenging due to the extent and size of transcoelomic metastatic spread within the abdomen. The largest mass observed was in the region of the pancreas and this prompted the initial presumptive diagnosis of a pancreatic neoplasm. However, this was later disproven by microscopic examination and immunohistochemistry of the liver which demonstrated rare transitioning of hepatocytes into neoplastic biliary ducts which is more consistent with a hepatic or biliary neoplasm. In either case, both primary hepatic and pancreatic neoplasms are rare in donkeys and horses.1,2,6
A retrospective study of post-mortem findings in donkeys with a mean age of 30.6 years (n=1444) from the UK, found that only 4.2% of those examined (60/1444) had malignant liver neoplasms (cholangiosarcoma or bile duct carcinoma) and none had evidence of pancreatic neoplasms.6 Similar findings were observed in a survey of donkeys diagnosed with neoplasia (n=125) in which 0.8% (1/125) were diagnosed with a primary hepatic neoplasm (biliary carcinoma) and none had pancreatic neoplasms.2 Pancreatic adenocarcinoma in donkeys has only been reported twice in the literature with the diagnosis primary based upon location and appearance; no immunohistochemistry was performed in these cases.3,7
Classification schemes of primary hepatic tumors are not available for donkeys or horses but have recently been established in canines.9 In general, primary liver neoplasms are characterized as either hepatocellular, cholangiocellular or neuroendocrine. Mature hepatocytes and cholangiocytes are the proposed origin of primary epithelial liver tumors; however cholangiolocarcinomas are believed to originate from hepatic progenitor cells and have hepatocellular, ductular and cholangiocellular characteristics.4,9 According to the canine classification scheme, cholangiolocarcinomas differ from cholangiocellular carcinomas morphologically due to the presence of central tubular structures surrounded by solid areas with the appearance of hepatic cords or acini.9 Furthermore, IHC patterns typical of canine tumors are distinct with tubular structures labelling positive for Keratin-19, EMA/MUC-1 and CD10 while the solid hepatocyte-like areas were positive for Keratin-19 and negative for HepPar-1, EMA/MUC-1 and CD10.
Though not a described entity in donkeys, this case most likely represents a cholangiolocarcinoma or less likely, a cholangiocellular carcinoma.
Contributing Institution:
Department of Biomedical Sciences
Ross University School of Veterinary
Medicine, P. O. Box 334; Basseterre, St. Kitts, West Indies
www.veterinary.rossu.edu
JPC Diagnosis:
Liver: Cholangiocellular carcinoma.
JPC Comment:
The final case of this conference is an interesting neoplasm with several great gross photos. From subgross magnification, conference participants quickly identified invasive behavior and neoplastic spread through the adjacent parenchyma, though evidence of lymphovascular invasion was less definitive. We repeated IHC for HepPar-1, CK7, and CK19 and performed PAS-Alcian Blue and Masson’s trichrome stains. IHC results were similar to the contributor – special stains highlighted mucus within neoplastic bile ducts and periductular fibrosis. Based on morphology, we favored a malignant epithelial neoplasm of biliary origin (i.e. cholangiocarcinoma).
The degree of fibrosis (scirrhous response) is fairly mild in this case compared with other cholangiocarcinomas. Dr. Cullen again highlighted the relationship of biliary epithelium, mesenchyme, and myofibroblasts that was explored in Case 1. Rather than a ‘disjointed conversation’ leading to inappropriate proliferation of bile ducts and connective tissue as was seen in ductal plate malformation, the phenotype in neoplasia is tilted in favor of collagen fiber deposition with ductular reaction being a minor feature.
Finally, Dr. Cullen weighed in on the idea of a cholangiolocarcinoma for this case. As the contributor hints at, exact definitions of this rare neoplasm are hard to come by with few published cases – as such, readers may benefit from a quick review of Komuta et al4 which has some excellent figures. MacSween's Pathology of the Liver (a veritable grail of hepatology) itself lacks a definition. Recently published human cases each differed in their microscopic descriptions of the tumor as well.5,7 The use of IHC is potentially challenging as ruling out metastatic adenocarcinoma may be complicated by lack of complete specificity. For example, many duct forming epithelia, as well as salivary epithelium may react with CK7 and select GI epithelial cells may be positive for HepPar-1. Hepatic progenitor cells may also variably express chromogranin A, similar to a biliary carcinoid. As such, we felt most confident that the neoplasm in this case had features supportive of a cholangiocarcinoma on H&E without IHCs to refute this interpretation.
References:
- Beeler-Marfisi J, Arroyo L, Caswell JL, Delay J, Bienzle D. Equine primary liver tumors: A case series and review of the literature. J Vet Diag Invest. 2010;22:174–183.
- Davis CR, Valentine BA, Gordon E, et al. Neoplasia in 125 donkeys (Equus asinus): literature review and a survey of five veterinary schools in the United States and Canada. J Vet Diag Invest. 2016;28:662–670.
- Kerr OM, Pearson GR, Rice DA. Pancreatic adenocarcinoma in a donkey. Equine Vet J. 1982;14:338–339.
- Komuta M, Spee B, Borght S, Vander, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544–1556.
- Makino K, Ishii T, Takeda H, et al. Integrated analyses of the genetic and clinicopathological features of cholangiolocarcinoma: cholangiolocarcinoma may be characterized by mismatch-repair deficiency. J Pathol. 2024 May;263(1):32-46.
- Morrow LD, Smith KC, Piercy RJ, et al. Retrospective Analysis of Post-Mortem Findings in 1,444 Aged Donkeys. J Comp Pathol. 2011;144:145–156.
- Sato N, Yamamura K, Oda E, et al. Cholangiolocarcinoma With Multiple Recurrences Successfully Treated With Repeated Liver Resection and Radiofrequency Ablation. Anticancer Res. 2020 Dec;40(12):7147-7153.
- Spanton JA, Mair TS, Krudewig C. Pancreatic adenocarcinoma in a donkey. Use of laparoscopy to aid the diagnosis. Equine Vet Educ. 2009;21:19–24.
- Van Sprundel RGHM, Van den Ingh TSGAM, Guscetti F, et al. Classification of primary hepatic tumours in the dog. Vet J. 2013;197:596–606.