Wednesday Slide Conference, Conference 15, Case 2
Signalment:
4-week- suckling piglet, male neutered, Swiss Large White, sus scrofa domesticus, porcine.
History:
In March 2023, an increase in spinal deformities was detected on a Swiss breeding farm, characterized by thoracic lordosis and lumbar kyphosis (“humpy-back syndrome”) and suckling as well as weaning piglets showed focal thickening of multiple ribs and facial oedema in an otherwise unremarkable clinical condition.
Gross Pathology:
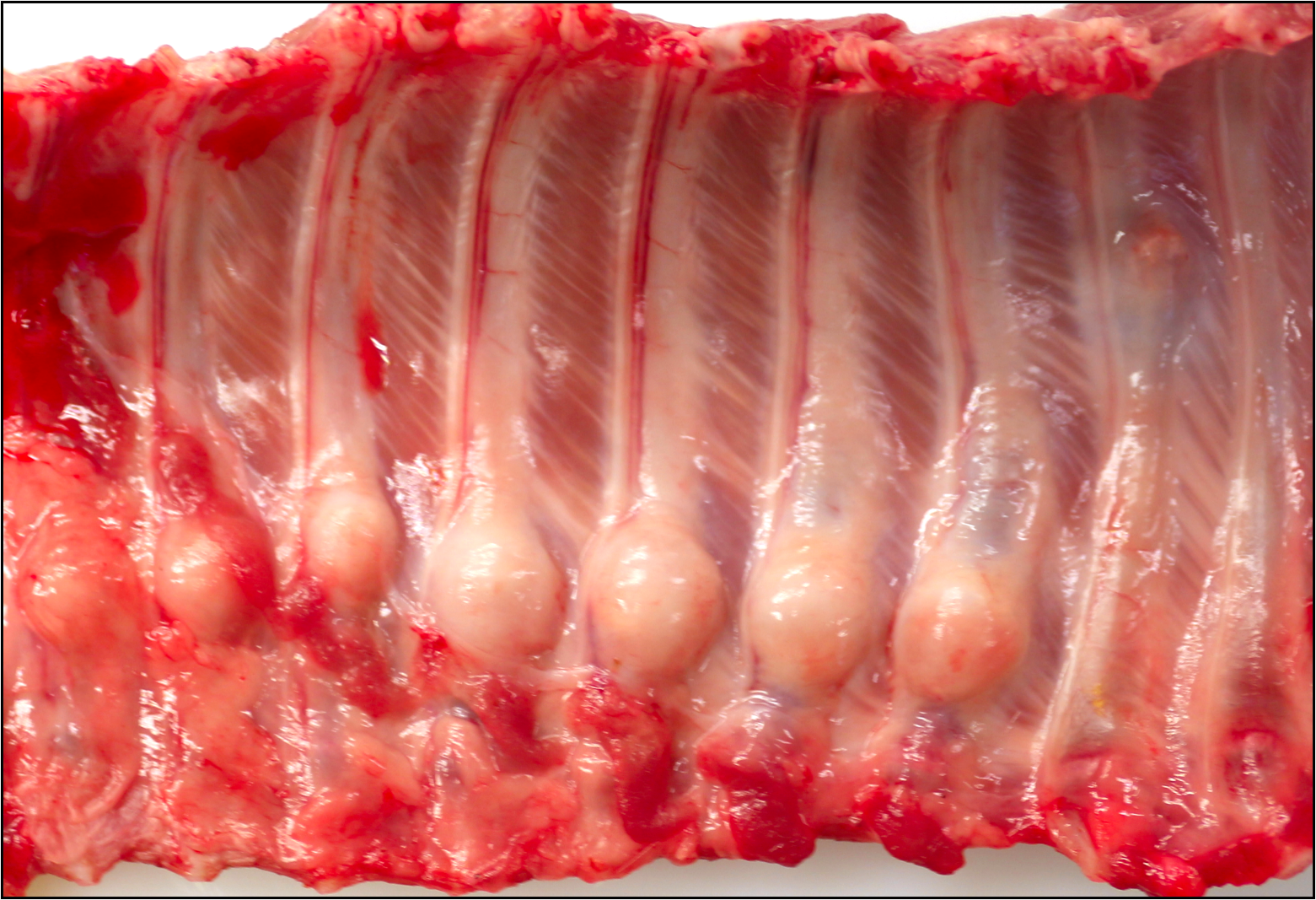
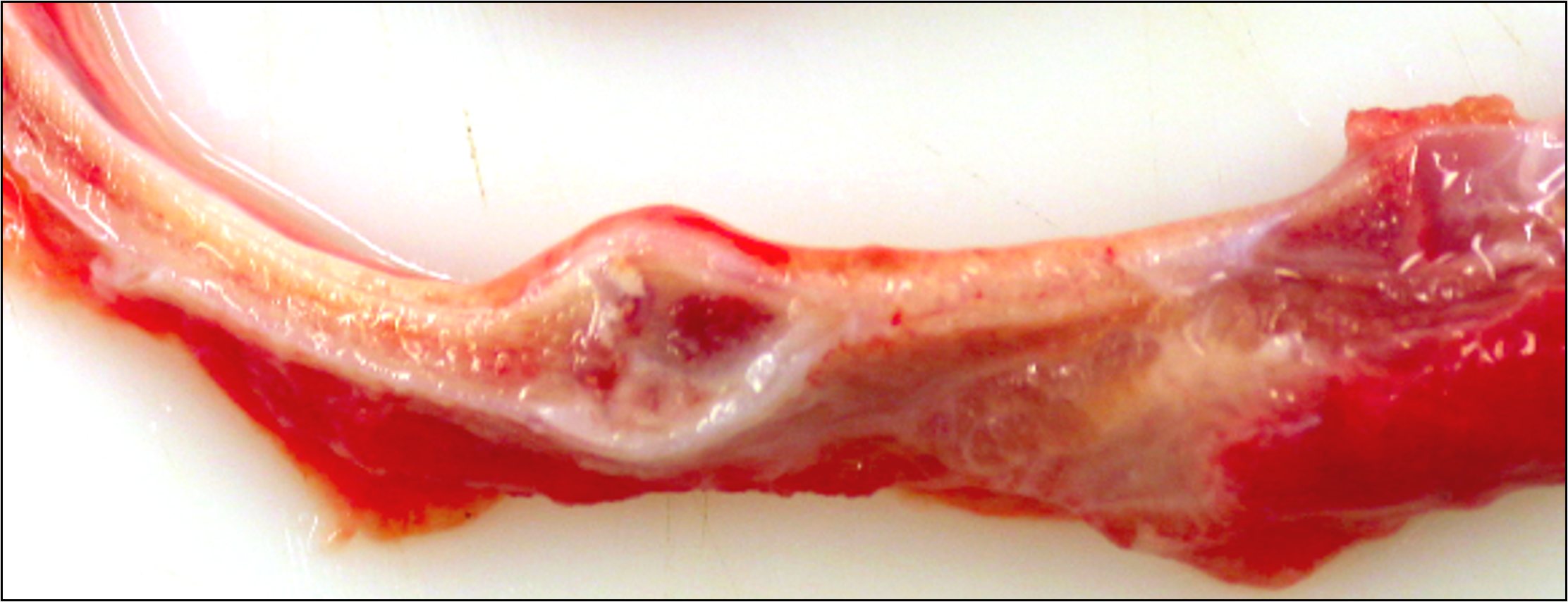
Seven adjacent ribs show a marked focal callus formation. The longitudinal sections reveal rib fractures.
Laboratory Results:
qPCR for PCV-3 revealed high viral loads in kidney (Ct 21), mesenteric lymph node (Ct 12), rib (Ct 26) and brain (Ct 23).
Immunohistochemistry for detection of PCV-2 yielded a negative result.
Microscopic Description:
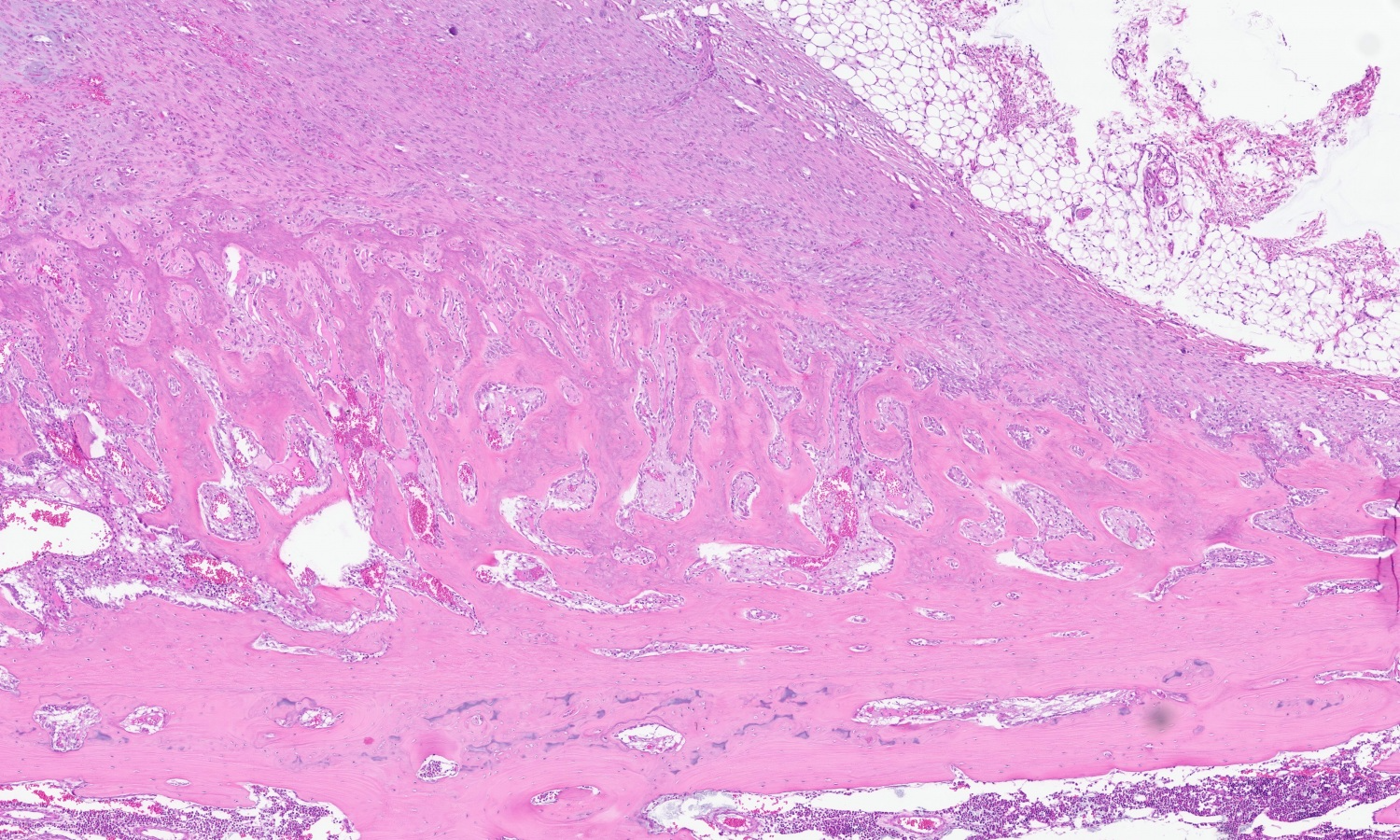
Bone, rib: In the calcification zone of the chondrocostal junction, a particularly high number of osteoblasts and osteoclasts are found (bone remodeling). Primary trabeculae vary in thickness, are fragmented and show cross-connections. The bone marrow contains all three hematopoietic cell lines with a slight increase in fibroblasts (myelofibrosis).
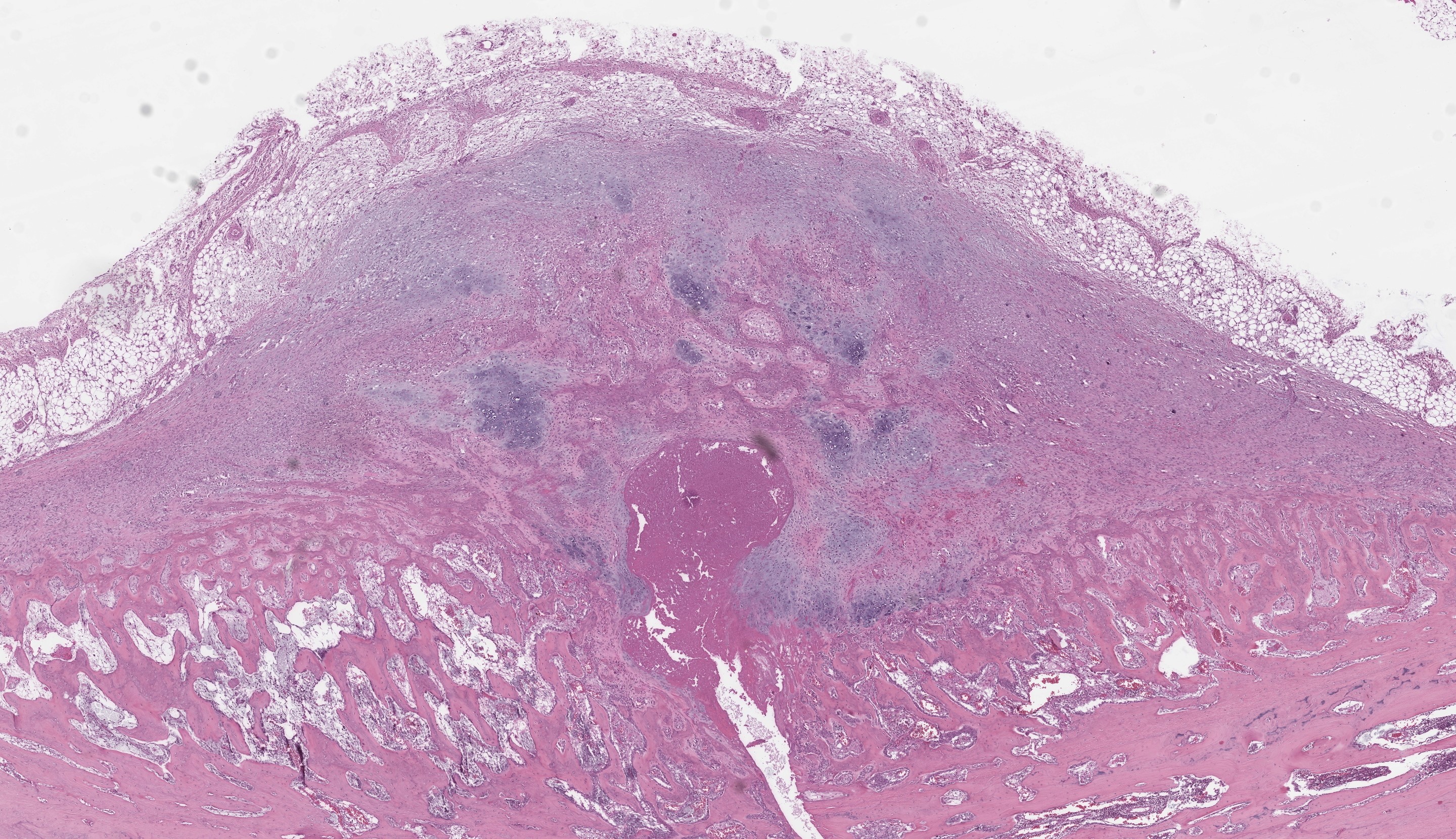
At the level of the macroscopically described bone distension, a blunt discontinuity of the osseous tissue is visible (fracture). The edges of the woven bone and multiple bone fragments within the fracture gap are lined by numerous multinucleated cells (osteoclasts) adjacent to scalloped, irregularly shaped bone (Howship’s lacunae). Within the fracture gap, the tissue is focally extensive replaced by irregular trabeculae of woven bone at varying stages of maturation along with granulation tissue. The immature woven bone, oriented perpendicular to the periosteum, is composed of densely organized collagen fibers and is often surrounded by numerous plump, large-nucleated mesenchymal cells (reactive osteoblasts) lying in a single layer. The granulation tissue extends into the adjacent bone marrow and is multifocally infiltrated by a moderate number of macrophages, lymphocytes, plasma cells, scattered neutrophils and few erythrocytes (acute hemorrhage).
In addition to multiple bone fragments, there are multifocal to coalescing areas of fibrous connective tissue and chondrocytes producing cartilage fragments (soft callus formation).
Furthermore, multifocal osteoid formations surrounded by osteoblasts are present. Neutrophils, fibrin and eosinophilic cellular and karyorrhectic debris (necrosis) as well as numerous multinucleated giant cells are found in the fracture gap.
Focally extending from the cortical surface and markedly elevating the periosteum, there is a periosteal proliferation of reactive, woven bone with trabeculae aligned perpendicular to the cortex (exostosis). The periosteum is diffusely highly expanded by collagenous connective tissue (periosteal fibrosis) and includes small fragments of osseous tissue and abundant extravascular erythrocytes (acute hemorrhage).
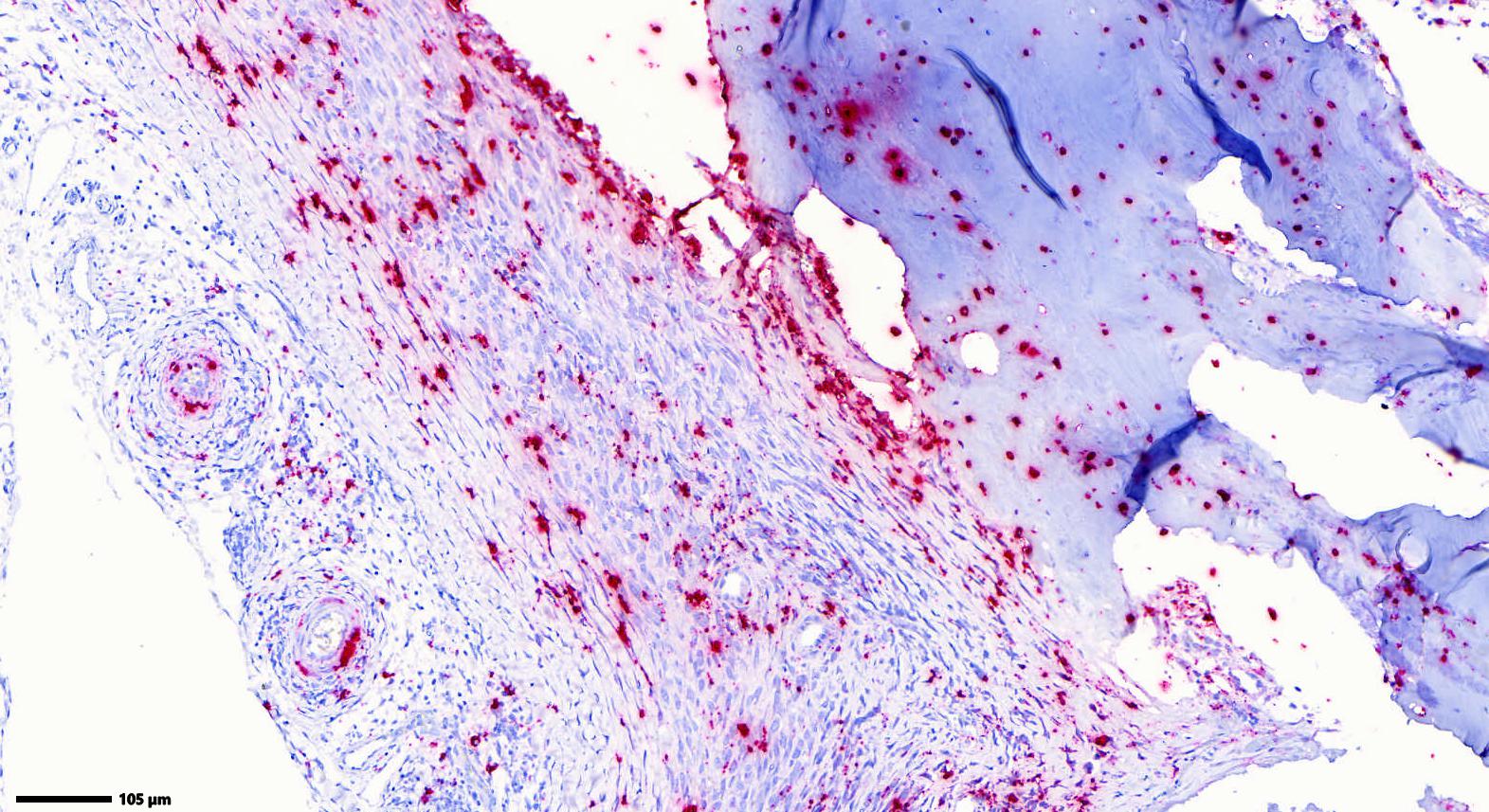
The arteries in the periosteal tissue and those in the adjacent intercostal muscles and adipose tissue show infiltration of the media and the adventitia by a moderate number of small lymphocytes, plasma cells and sporadic macrophages. Similar infiltrates are found periarterially. The venous and lymphatic vessels, as well as the nerves, do not exhibit any alterations. ISH detected abundant PCV-3 RNA in the rib (periosteal arterial walls, osteocytes and osteoblasts).
Contributor’s Morphologic Diagnosis:
Bone, rib: Fracture with bone remodeling, focally extensive, marked with reactive woven bone formation (fracture callus), myelofibrosis and hemorrhage, multifocal, acute, mild to moderate as well as exostosis, periosteal, circumferential, moderate.
Arteritis and periarteritis, lymphoplasmacytic and histiocytic, multifocal, moderate to severe.
Contributor’s Comment:
Circoviruses are single-stranded DNA viruses with a circular genome and one main capsid protein. They are prevalent across a variety of animals, including mammals, fish, birds, and insects. In pigs, four different porcine circoviruses (PCV) have been identified up to now: PCV-1, PCV-2, PCV-3 and PCV-4. PCVs are ubiquitous in global pig populations. While PCV-1 is accepted as non-pathogenic, PCV-2 is considered as an economically challenging pathogen on a global scale. Similarly to PCV-2, PCV-3 is widespread and detected in both healthy and diseased pigs, often in mummified and stillborn fetuses, indicating vertical and horizontal transmission of the viruses. PCV-4 has only recently been discovered and further information on this virus is required to understand its potential impact.1
PCV-3 infections are associated with various clinical and pathological manifestations, especially reproductive disorders, PDNS and multisystemic inflammatory diseases.2,4 Furthermore, viral DNA has been detected in asymptomatic pigs and coinfections comprised of PCV3 with other swine pathogens have been frequently reported. This suggests that PCV-3 may act as a cofactor for some pathogens or may need other cofactors to cause clinical signs of disease.3
In situ hybridization (ISH) is used for the detection of PCV-3 in lesions. Histopathologic expertise is crucial as associated lesions may be subtle, requiring a degree of confidence to recognize these conditions. To diagnose PCV-3, a histopathologic specimen including heart, lung, spleen and lymph nodes should be submitted.3 However, the clinical findings associated with PCV-3 are non-specific and require consideration of different pathogens.
The “humpy-back-syndrome” is earliest observed in pigs of 3 weeks but most often detected at an age of 8 to 16 weeks.4 It can arise as a secondary condition linked to multiple primary lesions within the vertebrae.5 Primary lesions include osteomyelitis, fractures, neoplasms and metabolic diseases.6 In addition, factors such as painful diseases of the legs and back7, Musculo-mechanical stress on the lumbar spine8, early onset of puberty in male pigs9 and intrauterine viral infections10 are contributing factors. A hereditary influence on the development of porcine kyphosis is suggested as well.10
The histological lesions in this case match the descriptions of PCV-3 systemic disease, but for the first time the virus has been detected by qPCR and ISH in bone lesions. In the last decade, authors reported cases of “humpy-back" pigs exhibiting histologically inflammatory vascular lesions comparable to those reported here.4,11 Therefore, pathomorphological investigations and possible detection of PCV-3 is recommended in pigs displaying bone lesions and “humpy-back” posture.
Contributing Institution:
Institute of Veterinary Pathology
Vetsuisse-Faculty
University of Zurich
Winterthurerstrasse 268
8057 Zurich
Switzerland
https://www.vetpathology.uzh.ch/de.html
JPC Diagnosis:
Bone, rib: Diaphyseal fracture with maturing callus.
JPC Comment:
This was a case that generated a lively discussion. As Dr. Cecere did his PhD on circoviruses, he guided the group through assessing the changes present in this slide which were descriptively rewarding (and quite detailed). Ultimately, we summarized this case in a simple way and chose to focus on the H&E features before considering the ISH (and question of causation) presented in this case.
We differed from the contributor in several key aspects. Foremost, the section that we reviewed lacked a significant vascular component, though there is mild lymphocytic perivasculitis and lymphocytic infiltration within peripheral nerves present. There is also a lack of overt necrosis. These features make it harder to assess the role of viral-induced insult as the cause of the weakening and eventual fracture of these ribs (i.e. through disruption of blood flow to the developing bone) as there is no evidence to support this interpretation. Likewise, we reviewed the ISH image supplied by the contributor (Figure 2-6) and agree that the localization of nucleic acid within the vessel wall comports with the expected behavior of PCV-3, though the signal also extends beyond osteocytes/osteoblasts to include numerous cells within the adjacent fibrous tissue and callus. Another potential interpretation is that the non-vascular signal represents transfer of circoviral nucleic acid sto antigen-presenting cells (e.g. infiltrating histiocytes). As such, this result raises the question of “correlation or causation” i.e., changes because of PCV-3 or in addition to PCV-3. Although not included with this submission, additional information about the spleen, kidney, liver, and/or peripheral lymph nodes to confirm the presence of circoviral-associated disease (especially with corroborating ISH) was suggested by Dr. Cecere to develop this hypothesis further in future cases. Conference participants were also skeptical of myelofibrosis in this piglet and interpreted mesenchymal cells as part of the developing callus to stabilize the fracture – by convention, myelofibrosis implies a primary defect which seems less likely in this case.
It is worth briefly discussing several of the slide features in weighing differential diagnoses for this case. As this was a very young piglet, it is important to know that the width of cortical bone closer to the physis/metaphysis (i.e., the “cut-back zone”) is typically thinner for physiological reasons (this is where elongation of the bone takes place) which does not reflect osteoporosis or osteopetrosis. This animal had not yet developed a secondary center of ossification as well. Similarly, the degree of cartilage present within the primary spongiosa is appropriate for the age of this animal. Comparing the hue of physeal cartilage to cartilage within the developing callus is helpful to determine if both growth plates are in section (they are not in the slides submitted to the conference). Conference participants also discussed the ‘rosary’ gross appearance (Figure 2-1) which resembles a rachitic (rickets) . In the submitted sections, the physis at the costochondral junction in this animal is relatively normal histologically.
References:
- Opriessnig T, Karuppannan AK, Castro AMMG, Xiao CT. Porcine circoviruses: current status, knowledge gaps and challenges. Virus Res. 2020 Sep;286:198044.
- Palinski R, Piñeyro P, Shang P,et al. A Novel Porcine Circovirus Distantly Related to Known Circoviruses Is Associated with Porcine Dermatitis and Nephropathy Syndrome and Reproductive Failure. J Virol. 2016 Dec 16;91(1):e01879-16.
- Kroeger et al. Five years of porcine circovirus 3: What have we learned about the clinical disease, immune pathogenesis, and diagnosis. Virus research, 2022, 314:198764
- Drolet et al. Alopecia areata and humpy-back syndrome in suckling piglets. The Canadian veterinary journal, 2012; 53(8):865-869.
- Nielsen et al. Juvenile kyphosis in pigs. A spontaneous model of Scheuermann's kyphosis. Journal of pathology, microbiology and immunology - the APMIS journal, 2005, 113(10).
- Lahrmann et al. Causes of kyphosis and lordosis with cuneiform vertebral deformation in swine. Berliner und Münchener Tierärztliche Wochenschrift, 1993, 106(4), 127-132.
- Lahrmann et al. Deformities of the ventral column in weanling pigs caused by hemivertebrae or block vertebrae. Journal of Veterinary Medicine Series A, 1990, 38, 691-695.
- Corradi et al. Acquired hemivertebrae in “humpy-backed” piglets. International Pig Veterinary Society Congress 2004, 1, 357.
- Done et al. Lordosis and kyphosis (“humpy-back”) in pigs. The pig journal, 1988, 41.
- Done. Lordosis and kyphosis (“humpy-back”) in pigs; a second type of the condition associated with hemivertebrae. The pig journal, 1999, 43, 148-153.
- Pallerés et al. Humpy backed pigs syndrome in spain. Anales de Veterinaria de Murcia, 2013, 29, 87-91.