Wednesday Slide Conference, 2025-2026, Conference 2, Case 3
Signalment:
3-year-old, male, African hedgehog, Atelerix albiventrisHistory:
The hedgehog was lethargic, with a history of decrease in water intake, respiratory distress, and anorexia. At clinical examination, the hedgehog was bradycardic, hypothermic, and with agonal breathing. Supportive care and treatment, including external warming, subcutaneous fluids and atropine, were given, without success. The hedgehog died of cardiac arrest and was submitted for postmortem examination.Gross Pathology:
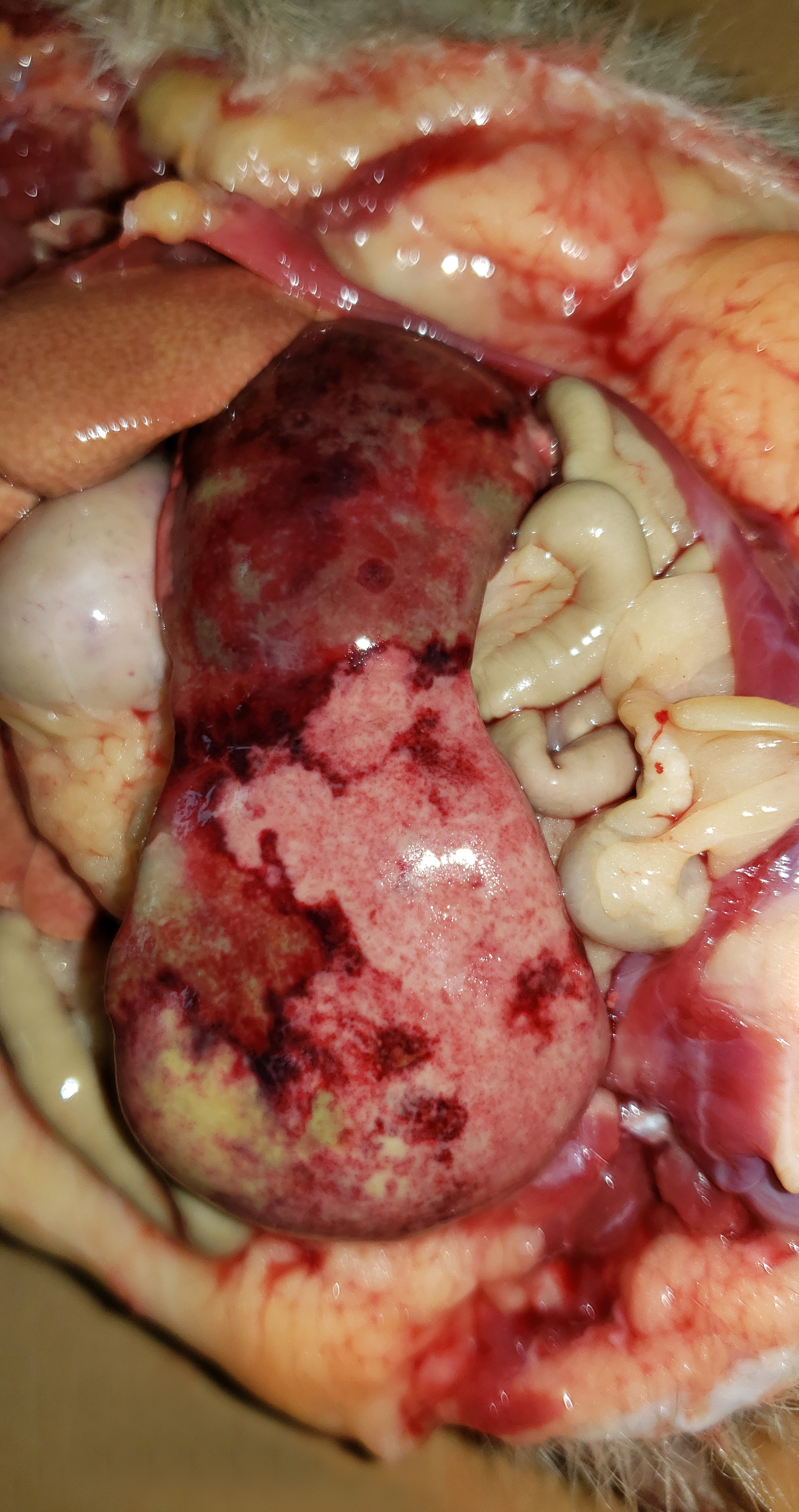
The hedgehog was in good body condition, with pale mucous membranes and distended abdomen. The spleen was severely enlarged, measuring 7.0 x 3.5 x 2.0 cm and weighing 6.52% of total body weight (reference interval: 0.1-2.47% of the body weight)3, with multifocal to coalescent pale yellow to green areas (necrosis) interspersed with dark red areas (hemorrhage). The liver was also enlarged, with round borders, diffuse pale-yellow discoloration, and accentuated lobular pattern . The liver samples floated in 10% formalin. The kidneys exhibited multifocal well delimited and irregular tan areas. In the distal region of the colon, a 2 mm diameter ulcer was observed in the mucosa, at 5 cm from the rectum.Laboratory Results:
N/AMicroscopic Description:
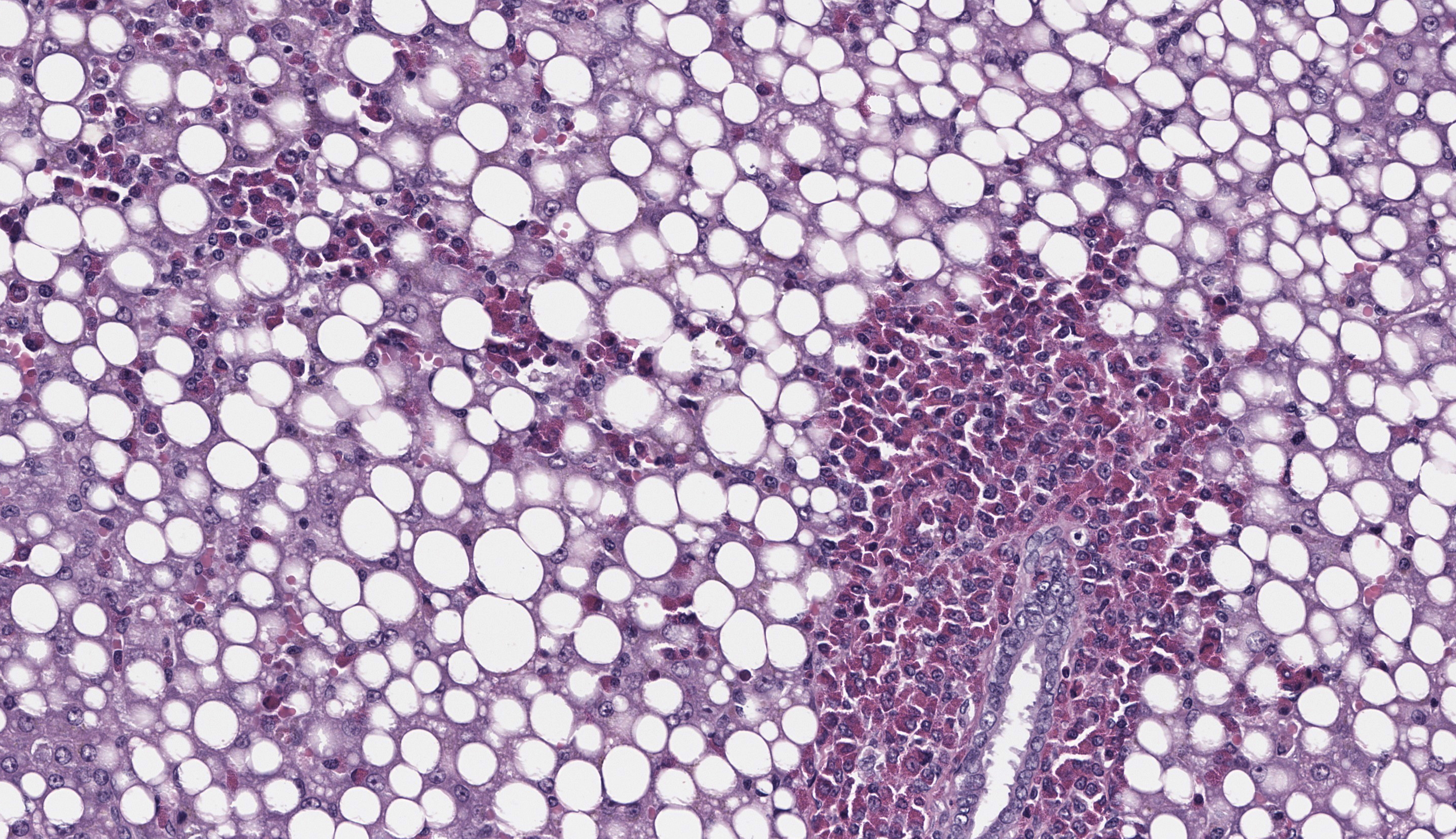
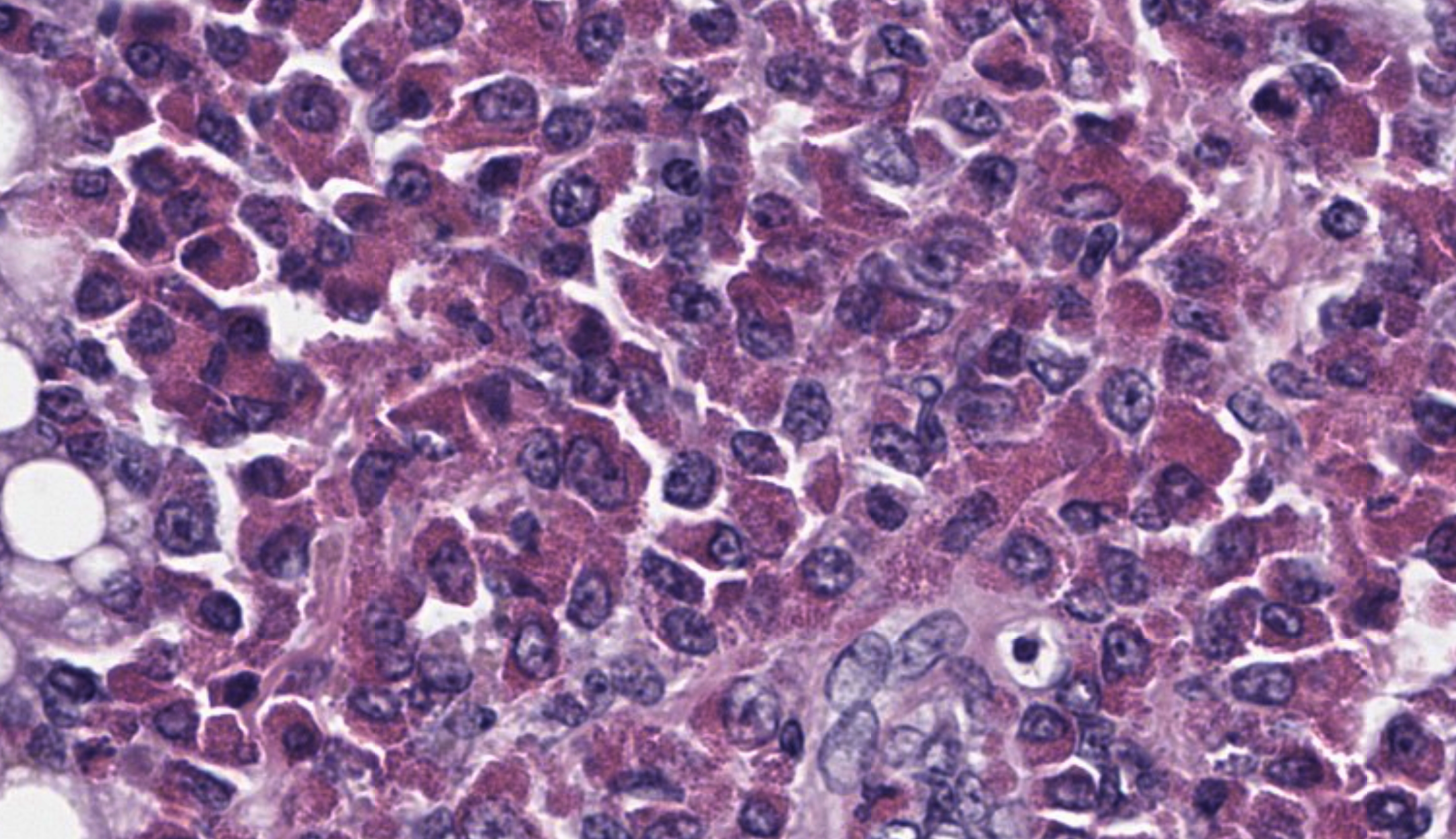
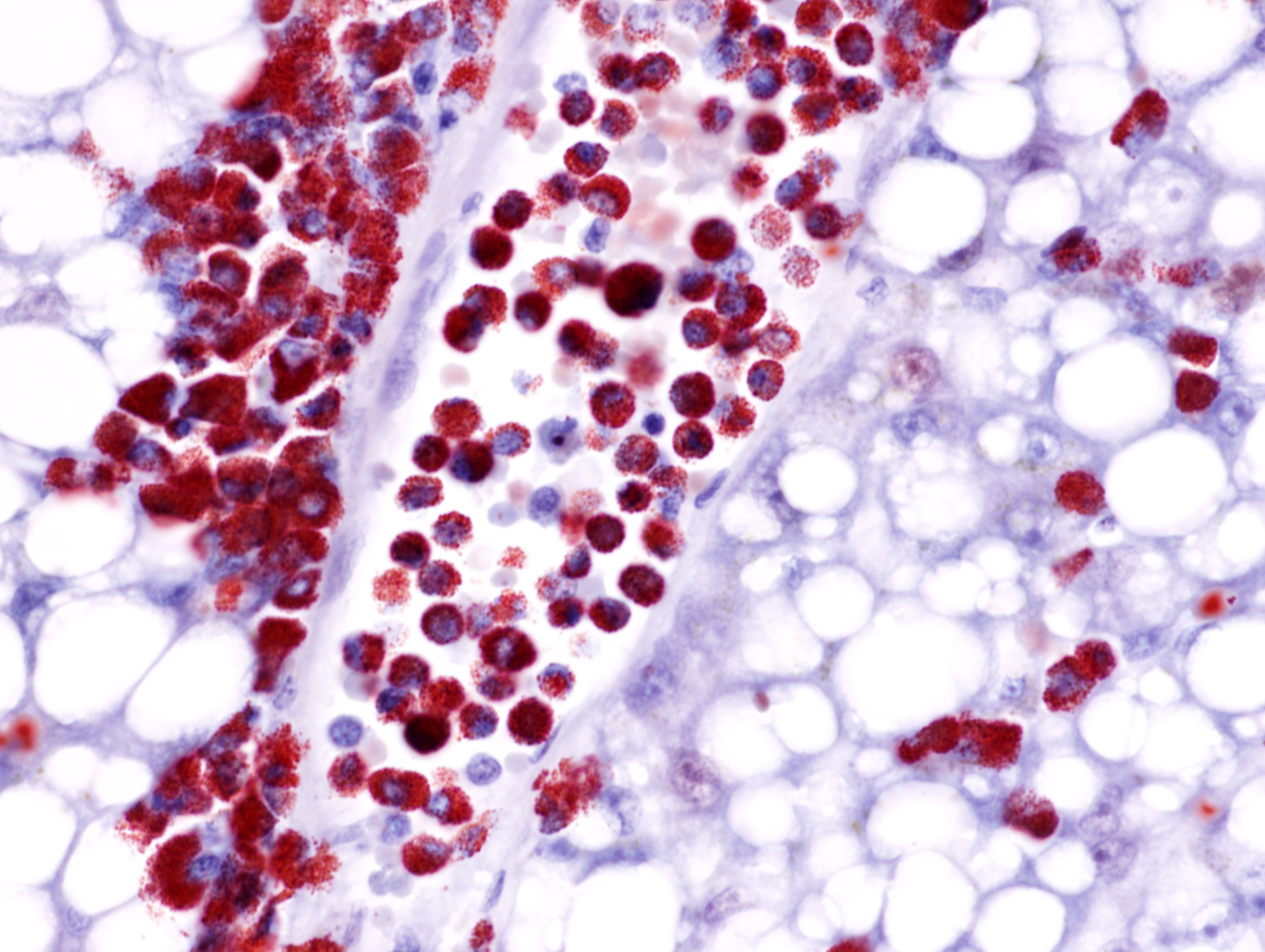
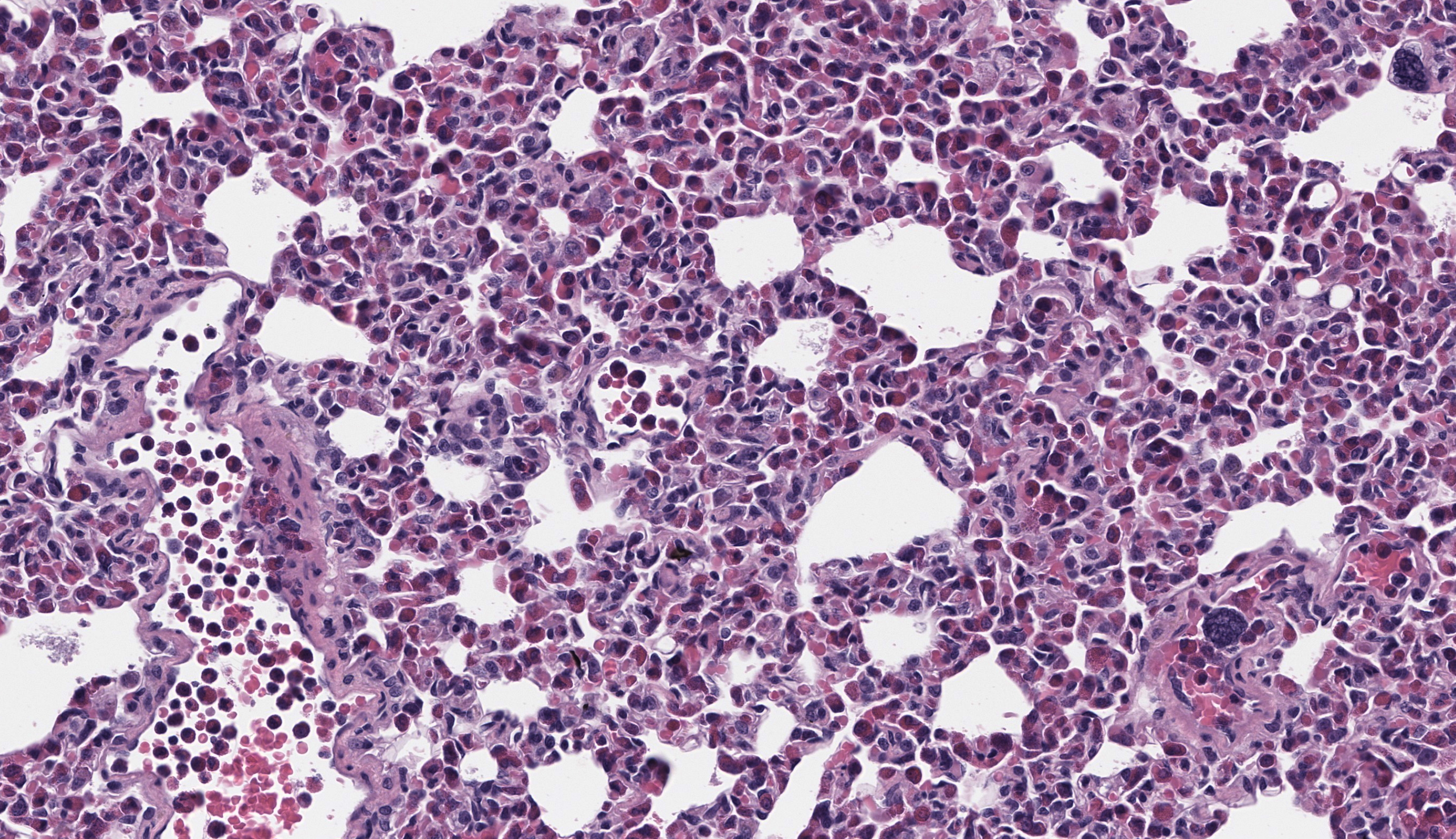
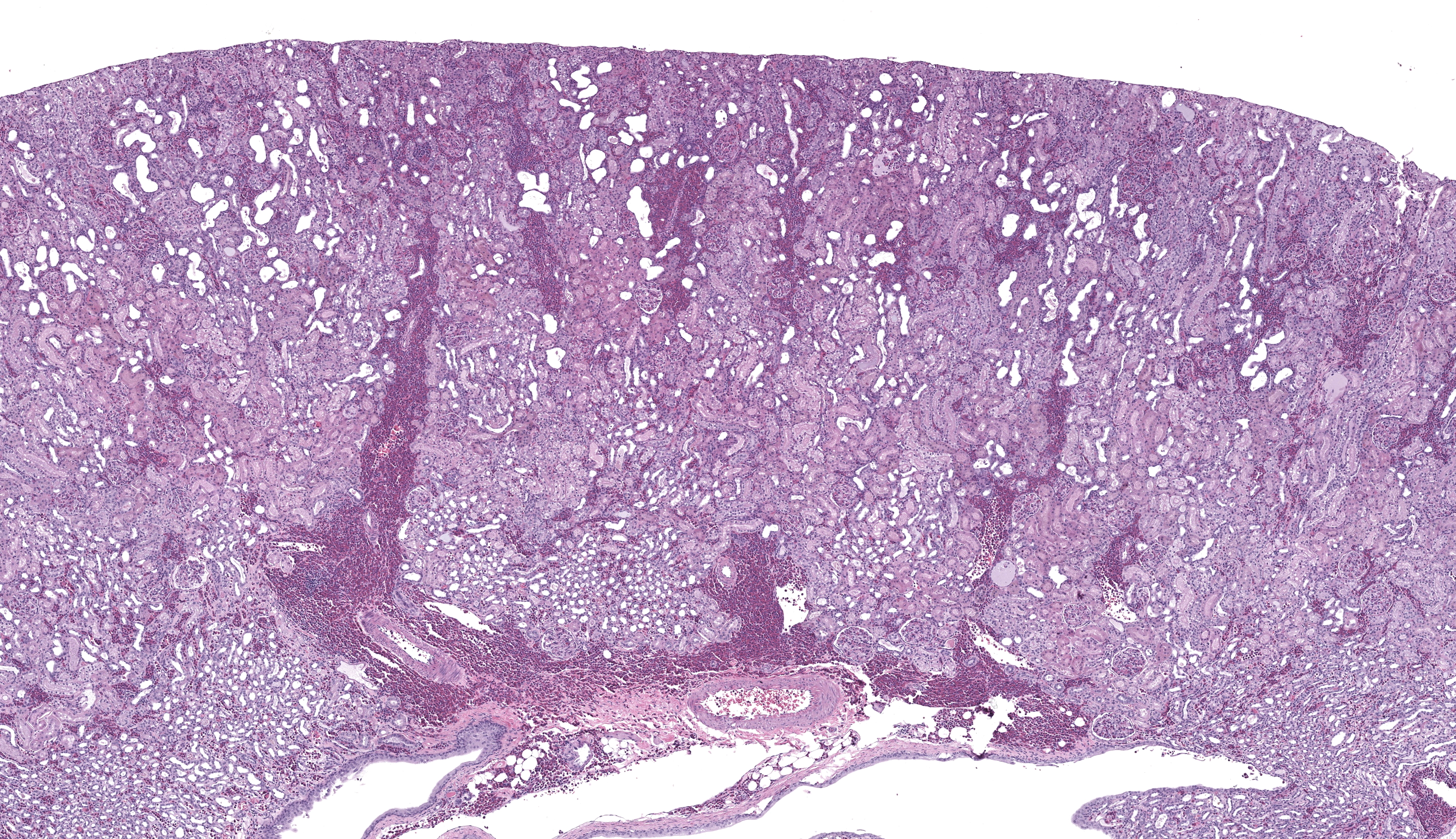
The submitted slide has a section of lung, a section of kidney, and a section of liver. In the lung, a neoplastic population of immature and mature eosinophils expands the peribronchiolar and perivascular interstitial tissue and is present in the lumen of blood vessels including both larger vessels and capillaries in the alveolar septa. The neoplastic cells are of intermediate size, measuring approximately 1.5 to 2 times the size of erythrocytes, and have abundant lightly basophilic cytoplasm containing variable quantities of small, round, eosinophilic granules. The nucleus of the immature eosinophils ranges from round to reniform, with open chromatin and one prominent nucleolus. The mature eosinophils have similar cytoplasm, multilobulated nuclei, and inconspicuous nucleoli. There is mild anisocytosis and anisokaryosis. Mitoses are rare (1 to 2 figures per high power field). The pleura is lined by mildly hypertrophic mesothelial cells. In the renal interstitium, there are multifocal to coalescing, predominantly perivascular sheets of immature eosinophils and fewer mature eosinophils like those described in the lung. A few immature eosinophils are entrapped in glomerular capillaries. The renal tubules have occasionally attenuated epithelium and intraluminal eosinophilic proteinaceous fluid or casts. Multifocal mineral deposits are present in renal medullary tubules. An aggregate of immature and mature eosinophils is seen in the perirenal adipose tissue. In the liver, hepatocytes are diffusely swollen, with clear cytoplasmic vacuoles (lipid-type vacuolar degeneration). The sinusoids are often expanded by predominantly immature and fewer mature eosinophils like those present in the lung and kidney. Perivascular areas (predominantly in the portal regions) have aggregates of similar cells, which also infiltrate multiple other tissues, including the bone marrow (sections not submitted). The cytoplasmic granules in both the immature and mature eosinophils stain strongly with the Luna stain.Contributor's Morphologic Diagnoses:
Lung, kidney, and liver: Eosinophilic leukemia Liver: Lipidosis, diffuse, severeContributor's Comment:
Spontaneous neoplasia is common in adult African hedgehogs without sex predilection. The median age of affected animals is 3.5 years.11 The most common sites of tumor development in these animals are skin (especially mammary gland), lymphoid, gastrointestinal, endocrine, and reproductive systems. Most of these tumors are malignant and tend to have a poor prognosis.11 Hematopoietic tumors can account for up to 11% of the tumors in hedgehogs. Lymphoma is the most common of this type of tumor, predominantly of the multicentric and alimentary forms, while leukemia in hedgehogs is considered rare.9,11 Leukemias in hedgehogs include eosinophilic leukemia and acute leukemia/lymphoma.7,9,10Eosinophilic leukemia is a variant of granulocytic leukemia and has been described in cats and humans.3,4 This type of neoplasm is considered to be chronic with an indolent course, and the cells are usually immature with eosinophilic differentiation. Some reports suggest that hedgehogs may have a unique genetic susceptibility to this specific type of leukemia, and that the behavior of the neoplasm is more aggressive in this species.9 Differential diagnoses include idiopathic hypereosinophilic syndrome and paraneoplastic hypereosinophilia, both described as increase of solely mature eosinophils in blood and tissues.4,7,9 The diagnosis of idiopathic hypereosinophilic syndrome is made by exclusion, in the absence of an eosinophilic neoplasm, like leukemia, or by determining primary causes of reactive increase of eosinophils, such as allergies, autoimmune disease, or parasitic infestation.7,9,10
The diagnosis of leukemia can be performed through clinical pathologic findings, especially marked leukocytosis, composed mostly of immature eosinophils, which can be observed in the peripheral blood smear. Animals with myelogenous leukemia can present concurrent cytopenias, like non-regenerative anemia and thrombocytopenia. Serum biochemistry findings depend on the organs affected by the neoplastic cells. The literature describes a predominance of liver injury, leading to hypoalbuminemia.9 Histologically, it is common to visualize neoplastic cell infiltrate in multiple organs, including the bone marrow, pancreas, liver, kidney, gastrointestinal tract, lungs, lymph nodes, heart, and spleen.7,9 Although eosinophils can be easily recognized cytologically or histologically, the identification of immature cells can be a challenge. Histochemistry and/or immunohistochemistry may be helpful in these cases to confirm the lineage of the leukemia cells. In cases of eosinophilic leukemia, Luna stain is the most consistent stain utilized to highlight the granules of both immature and mature eosinophils.10 Cytochemical staining for myeloperoxidase (MPO), alkaline phosphatase (ALP), and Sudan Black B can also be performed to demonstrate the granules of eosinophils in blood smears.9
In the current case, variable degrees of neoplastic cell infiltrate were observed in the bone marrow, lungs, trachea, nasal cavity, heart, kidneys, liver, spleen, pancreas, gastrointestinal tract, mesenteric lymph node, skin, eyes, eyelids, lacrimal glands, sciatic nerve, and cerebral meninges. Blood vessels were filled with immature eosinophils, which indicates abundant neoplastic eosinophils were in circulation, consistent with leukemia. Causes of hypereosinophilia, such as parasitism or allergy, were ruled out based on the history and lack of gross or microscopic evidence. The severely enlarged spleen (6.52% of total body weight; reference interval: 0.1-2.47% body weight)5 had multifocal to coalescent, large areas of necrosis within extensive sheets of neoplastic cell infiltrate; little of the normal parenchyma remained. The bone marrow was markedly hypercellular; 70-80% of the cellularity was composed of immature forms of eosinophils, with 2 mitotic figures per 10 high power fields. Megakaryocytes and erythroid precursor cells were markedly reduced in numbers. The cause of death was presumed to be from multiorgan dysfunction due to the severe neoplastic cell infiltrate in all organs, further complicated by the severe hepatic lipidosis.
There is no scientific evidence of the efficacy of chemotherapy for the treatment of eosinophilic leukemia in hedgehogs. Drugs like cytarabine, used for the treatment of cats and dogs with hypereosinophilic syndrome, were tested in hedgehogs.10 No treatment has been successfully established for this condition in hedgehogs, however. Affected animals usually die shortly after diagnosis.8-10
Contributing Institution:
Louisiana Animal Disease Diagnostic, Laboratory (LADDL), School of Veterinary, Medicine, Louisiana State University (http://www1.vetmed.lsu.edu/laddl/index.JPC Diagnoses:
- Liver, kidney, lung: Myeloproliferative neoplasm, favor chronic eosinophilic leuke-mia.
- Liver, hepatocytes: Lipidosis, diffuse, severe.
JPC Comment:
Of the four entities seen in today’s conference, this is the only one that has been seen in a Wednesday Slide Conference before (WSC 2020-2021, Conf 19, Case 2). In what initially was thought to be a straightforward case, some conference participants engaged in a spirited debate about whether to call this acute vs chronic eosinophilic leukemia and if it also classified as an eosinophilic sarcoma due to extravasation of the neoplastic epithelial cells into the surrounding tissues. As such, a CD34 immunohistochemical marker was performed post-conference to determine maturity of the eosinophils, which revealed that the neoplastic cells were not immunoreactive to CD34. This allowed participants to conclude that the leukemic portion of the diagnosis was chronic. Additionally, this case was sent for human hematopathology consult with the Walter Reed National Military Medical Center, who expressed that they would diagnose this case as chronic eosinophilic leukemia based on the degree of granularity of the eosinophils, which they stated is a feature of maturity. However, they also mentioned that “myeloproliferative neoplasm” would be a more appropriate term according to the WHO, as there was no clinical documentation to demonstrate a peripheral eosinophilia over time in this patient and there was no clonality performed. Both of these are considered diagnostic criteria according to the most updated World Health Organization classification for leukemias.2 All factors considered, conference participants agreed with the diagnosis of “myeloproliferative neoplasm, favor chronic eosinophilic leukemia” in line with the WHO classification.The eosinophil was discovered by Paul Erlich in 1879 when he found distinctive properties of a particular subset of leukocytes that exhibited a pink color when stained with eosin dye.1 Eosin dye is made from fluorescein, which is a coal tar derivative, that is treated in an ethanol solution with bromine to form tetrabromofluorescein.8 This compound, which is acidic, is then neutralized into a salt, resulting in a water (or ethanol) soluble dye. Eosin is negatively charged, and thus stains positively charged tissues, including cytoplasmic elements, extracellular matrix, and connective tissues such as collagen.8 Additionally, it stains basic proteins, including Major Basic Protein found in the granules of eosinophils, which is what causes them to stain so strongly with eosin and gives them their characteristic name.8
Eosinophils originate in the bone marrow from multipotent hematopoietic stem cells (HSC) that differentiate down the myeloid lineage. The common CD34+ myeloid progenitor (CMP) gives rise to myeloblasts, which are enter granulopoiesis towards one of three cell types: neutrophils, basophils, and eosinophils. The differentiation and maturation of eosinophils through eosinophilic lineage are dependent on timely expression and presence of several transcription factors and cytokines, such as interleukin-5 (IL-5).12 IL-5 is the most important factor promoting eosinophil differentiation, maturation, and survival. Other cytokines, including IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF) also contribute to this process.12
Mature eosinophils contain granules rich in major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil peroxidase (EPO/EPX), and eosinophil-derived neurotoxin (EDN), among other things.12 Eosinophil granules are cytotoxic and are released in response to a variety of infections, particularly parasitic infection, as well as to allergen exposure. Normally, in response to infection or allergen, Type 2 inflammatory (Th2) cells that are in proximity to the stressor produce high levels of IL-5, which triggers eosinophil infiltration into tissues and activation.12 Activated eosinophils release a variety of substances, including cytokines, chemokines, and granule proteins such as those mentioned above. These mediators, if not regulated, contribute to the tissue damage and dysfunction seen in the affected organs. Eosinophil peroxidase, in particular, impart a characteristic green hue to tissues it affects due to it’s high iron concentration, which at one point led to the use of the now-retired terms “chloroleukemia” and “chloroma” to describe associated lesions of eosinophilic leukemias grossly.
Now that some basics eosinophil origin and function have been discussed, what about eosinophilic leukemias? Specific mutations in the CD34+ myeloid progenitor cells can lead to primary eosinophilic disorders, including leukemias and hypereosinophilic syndromes, where eosinophils are part of the clonal expansion. The FIP1L1-PDGFRA fusion gene is a well-studied example of such a mutation associated with chronic eosinophilic leukemia (CEL) in humans.6 This gene is not normally present and arises from a chromosomal deletion that results in the production of an activated tyrosine kinase that drives uncontrolled growth and division of myeloid cells, particularly eosinophils.6
In veterinary species, this disease is rare with only scattered reports in African pygmy hedgehogs, cats, dogs, and one report in a tiger salamander. Despite the growing body of information that exists about this entity in humans, studying the lineage and genetics of eosinophilic leukemia in veterinary species has proven to be challenging due to the lack of cross-reactive or species-specific reagents and immunohistochemical markers.9 Until that changes and someone develops species-specific reagents to study eosinophilic leukemia in African pygmy hedgehogs (or other affected species, I’m sure there’s a Ph.D. niche there), further understanding of this disease in animals will come with time....and funding.
References:
- Aoki A, Hirahara K, Kiuchi M, Nakayama T. Eosinophils: Cells known for over 140 years with broad and new functions. Allergol Int. 2021;70(1):3-8.
- Barbui T, Thiele J, Gisslinger H, Kvasnicka HM, Vannucchi AM, Guglielmelli P, Orazi A, Tefferi A. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018;8(2):15.
- Bain BJ, Fletcher SH: Chronic Eosinophilic Leukemias and the Myeloproliferative Variant of the Hypereosinophilic Syndrome. Immunol Allergy Clin.2007;27:377-388.
- Gelain ME, Antoniazzi E, Bertazzolo W, Zaccolo M, Comazzi S: Chronic eosinophilic leukemia in a cat: cytochemical and immunophenotypical features. Vet Clin Pathol. 2006;35:454-459.
- Girgiri IA, Gambo BG, Ibrahim B, Bwala A: Morphometric studies of some visceral organs and gastrointestinal tract of fourtoed African hedgehog (Atelerix albiventris). J Morphol Sci. 2015;32(1):29-32.
- Haydon SJ, Ross DE, Caucci S, Clark S, Hrinczenko B. Chronic Eosinophilic Leukemia Positive for FIP1L1-PDGFRa. Cureus. 2025;17(2)
- Higbie CT, Eshar D, Choudhary S, Pohlman LM, Ganta CR, Andrews G: Eosinophilic leukemia in a pet African hedgehog (Atelerix Albiventris). J Exot Pet Med. 2016;25:65-71.
- Kay AB. The early history of the eosinophil. Clin Exp Allergy. 2015;45(3):575
- Koizumi I, Hernandez-Muguiro D, Chu SAA, Stokol T, Asakawa MG: Clinicopathologic findings of spontaneous leukemia in 9 pet African hedgehogs (Atelerix albiventris). Front Vet Sci. 2020;7(54):110.
- Martinez-Jimenez D, Garner B, Coutermarsh-Ott S, et al. Eosinophilic leukemia in three African pygmy hedgehogs (Atelerix albiventris) and validation of Luna stain. J Vet Diag Invest. 2017;29(2):217-223.
- Raymond JT, Garner MM: Spontaneous tumors in captive African hedgehogs (Atelerix albiventris): A retrospective study. J Comp Pathol. 2001;124:128-133.
- Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol.2013;13(1):9-22.