Signalment:
2.5-year-old male Lined Seahorse,
Hippocampus erectusThis seahorse initially presented for a 3 week history of multiple bubbles on the tail. No changes in behavior or appetite were noted. The three other seahorses in the aquarium were unaffected. All four seahorses were kept in an indoor 40 gallon glass hexagonal tank with 60 lbs of live rock, crushed coral and sand substrate, and sea grass. The water temperature was kept at 67o F. Tap water was used to make salt water. Water quality was maintained by a large filter, protein skimmer and a 20-30% water change every 2 weeks. The aeration unit was a pump with a submerged airstone. The diet was composed of frozen shrimp. On physical exam, the seahorse had inappropriate orientation with its tail floating above the head. At the distal half of the tail, there were five randomly scattered regions where the skin was lifted above a gas-filled space (bubble). Two cavities were aspirated for culture and the rest were reduced with a #28 needle. Bacterial culture from one aspirate was negative and from the other aspirate, small numbers of mixed growth grew including fastidious gram-negative bacteria. No bacteria or protozoa were seen on skin scrapes. A diagnosis of internal gas bubble disease was made and the patient was discharged on ceftazadine (20mg/kg, IM, every three days). Two weeks after the initial presentation, the seahorse was reevaluated. At this time there were more and larger bubbles on the tail, the seahorse was floating upside down, and was no longer feeding. One of the other seahorses in the tank had begun to show similar signs. The seahorse and 3 cohorts were treated with ceftazadine (20mg/kg, IM, every three days). A week later the seahorse was euthanized at home by the owner.
Gross Description:
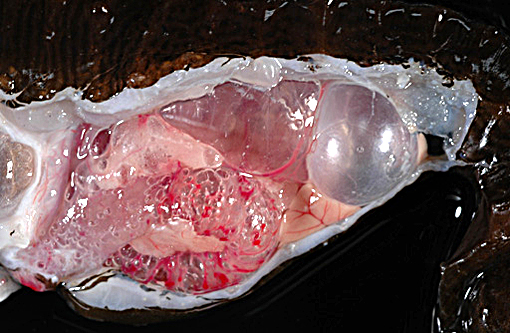
The adult male lined seahorse weighed 20.7 g, was in good post-mortem condition and had adequate adipose stores. Five subcutaneous gas-filled bubbles were present on the ventral aspect of the distal half of the tail, measuring (cranial to caudal): 0.4 x .03 x 0.2 cm, 0.6 x 0.5 x 0.4 cm, 0.5 x 0.4 x 0.3 cm, 0.5 x 0.5 x 0.4 cm, and 1.1 x 0.6 x 0.5 cm. The swim bladder was severely distended and displaced the esophagus and stomach ventrally and compressed the kidneys dorsally. The intestine was markedly distended and displaced the liver ventrally and to the left.
Histopathologic Description:
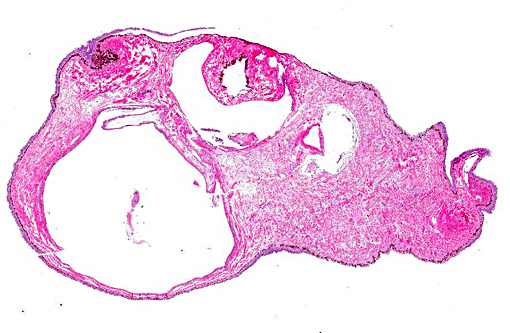
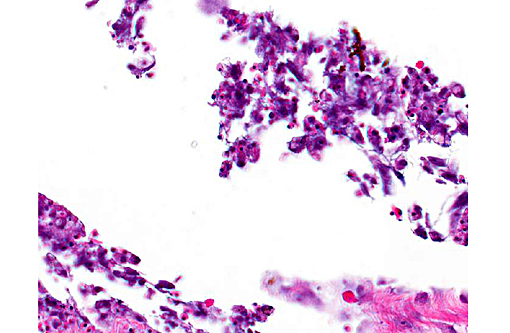
In the examined longitudinal section of the tip of the tail, multiple 1 to 5mm clear spaces surrounded by variable amount of inflammation expand and compress the dermis, subcutaneous tissue and skeletal muscle. The clear spaces are variably lined by flattened elongated cells (presumed fibroblasts) or a combination of histiocytes, lymphocytes and infrequent multinucleate giant cells. Most spaces are empty. Less than 10% of the spaces contain scattered, radiating aggregates of eosinophilic, thin (<1μm diameter) up to 10 μm in length, beaded, filamentous bacteria that are surrounded by a 2-5 cell-thick rim of macrophages. In the deep dermis and subcutis between the clear spaces, there are dense, similar inflammatory cells that surround dilated blood vessels and are intermixed with haphazardly arranged plump fibroblasts. Focally, the inflammation extends to a central fragment of vertebral bone that has an irregular, scalloped surface lined by osteoclasts (bone resorption). Multifocally the epidermis is hyperplastic.
Morphologic Diagnosis:
1. Tail (dermis, subcutis, skeletal muscle): Multifocal gas bubbles with chronic granulomatous cellulitis, and focal boney remodeling.
2. Subcutis: Granulomatous cellulitis with intalesional gram-positive filamentous bacteria
Lab Results:
No bacterial organisms were seen on direct smear nor cultured from a coelomic cavity swab or liver sample.
Condition:
Gas bubble disease, seahorse
Contributor Comment:
Gas bubble disease (GBD) is a traumatic condition that can generically be referred to as a gas entrapment disorder.(3) The obvious gross lesions, gas-filled bubbles in the tissue, give the entity its name. In Syngnathids and sea horses in particular, gas bubble disease is a classic and frequently recognized entity.(1,2) In a 2005 review of syngnathids in public aquaria,
H. procerus, H. ingens, H. subelongatus and
H. fisheri were reported to be unusually susceptible to developing gas bubble disease.(2) Though susceptibility is more likely defined by housing and husbandry conditions rather than species susceptibility,
H. fisheri is considered not suited for long-term display in aquaria because its particular susceptibility may be due to a need for access to deeper waters in order to maintain homeostasis.(2) Common presentations include gas entrapment in the brood pouch of male seahorses, subcutaneous emphysema, over-inflation of the swim bladder, and/or abnormal swimming, body posture or loss of neutral buoyancy. Subcutaneous emphysema most commonly presents as grossly visible bubbles in the tail and along with brood pouch over-inflation, are the most frequently observed gas entrapment problems.(1,2) Treatment of this disorder includes direct aspiration of the bubbles and administration of a carbonic anhydrase inhibitor, acetazolamide, and/or an antibiotic, ceftazidime.(1) Some institutions have had temporary success in reducing gas bubbles by increasing the barometric pressure (dropping the seahorses to a depth of 4 meters).(1) To date, the association between gas super-saturation and/or infectious agents and the development of gas bubble disease in seahorses has not been proven.(1,2)
The rare published reports of the histopathologic correlates have described gas-filled pseudocysts surrounded by varying amounts of fibrosis and granulomatous to histiocytic inflammation.(2) (personal communication, A. Nyaoke) No infectious organisms, unlike in this case, were described in association with the lesions. In this case, we suggest that the filamentous bacteria were an opportunistic infection, potentially introduced during the needle-aspirate reduction of the gas filled spaces and may explain the success of using antibiotics as an adjunct therapy to gas-reducing methods. In support of this theory, the deeper gas filled spaces, such as those within the muscle, did not contain bacteria. The filamentous bacteria were not recovered on culture, likely due to sampling site (coelomic cavity and liver), and were gram-negative and Fites acid-fast negative.
In general, in teleost fish and other aquatic species, gas bubble disease is associated with supersaturation of the water with nitrogen or oxygen and can be caused by anything that alters the gas saturation of the water, such as leaks in the pump or valve systems, sudden temperature gradients, altitude changes during air transportation and/or rapid barometric changes most often associated with collection of specimens at depth.(3) The disease is particularly important in cultured fish and amphibians;(4,5) however, wild fish are occasionally affected with reported cases in several fresh and marine species.(3) Some of the cases in the wild have been associated with heavy macroalgal blooms that were considered responsible for excessive levels of dissolved gases and subsequent disease. Fish often die of GBD without overt clinical signs. Occlusion of the large branchial vessels by gas emboli with endothelial damage and thrombi formation is considered the principal cause of acute mortality.(3,6) When clinical signs are present in teleost fish, they range from bilateral exophthalmia, subcutaneous emphysema, gasping behavior, hemorrhages, and anomalous swimming or loss of neutral buoyancy.(3,5)
JPC Diagnosis:
1. Tail, skeletal muscle: Cavitary pseudocyst, consistent with gas bubbles in tissue.
2. Tail: Cellulitis, granulomatous, chronic, multifocal, with granulation tissue and numerous bacilli.
Conference Comment:
This is an intriguing case that initiated the exchange of theories among conference participants regarding the origin of the cysts in this seahorse, as there seemed to be three varieties present. The first occurring most prominently are the empty lumens lined by a layer of inflammatory cells which most believed to be gas-filled pseudocysts within the subcutaneous tissue. Occasional cysts in most sections appeared to be lined by endothelial cells leading some to conclude they were gas-distended lymphatics. Finally, as the contributor mentioned, the inflammatory infiltrate surrounded filamentous bacteria and, in some sections, encompassed much of the cystic space lending credence to speculation these were either primary granulomas or secondary bacterial infections. The hypothesis proposed by the contributor is quite plausible given the history of aspiration in this case.
In rare slides, there is a section of bone likely representing the vertebrae of this animal. When present, the bone edges are scalloped and often lined by activated osteoclasts consistent with active bone remodeling. This seems to provide further evidence in support of a chronic bacterial infection, which may correlate with the initial positive bacterial culture of this area.
Considerable information has been derived from the lifecycle of seahorses following the development of commercial rearing facilities. They are peculiar creatures with the responsibility of fertilization and incubation of the young taken on by the males following a monogamous relationship with a single female. In addition to vibriosis and mycobacteriosis, gas entrapment problems are a major health issue for seahorses in culture and their relationship with gas supersaturation and infectious agents is largely unresolved.(1)
References:
1. Koldewey HM-S, K.M.: A global review of the seahorse aquaculture. Aquaculture 302: 131-152, 2010
2. Koldewey H: Syngnathid Husbandry in Public Aquariums, London, 2005
3. Roberts JR: Fish Pathology, Third ed. Harcourt Publishers Limited, London, 2001
4. Saeed MO, al-Thobaiti SA: Gas bubble disease in farmed fish in Saudi Arabia. Vet Rec 140: 682-684, 1997
5. Salas-Leiton E, Canovas-Conesa B, Zerolo R, Lopez-Barea J, Canavate JP, Alhama J: Proteomics of juvenile senegal sole (Solea senegalensis) affected by gas bubble disease in hyperoxygenated ponds. Mar Biotechnol (NY) 11: 473-487, 2009
6. Speare DJ: Endothelial lesions associated with gas bubble disease in fish. J Comp Pathol 104: 327-335, 1991