Signalment:
10-year-old, male, Boxer dog, canine (
Canis familiaris)This dog presented with a one-week history of ataxia, general weakness and occasional temporary loss of consciousness. Neurological examination showed cranial nerve reflexes within normal limits. Two to three hours later, the animal had lost the sense of smell, palpebral and swallowing reflexes, perception of painful stimuli, and was blind. Two hours later the patient was in a coma. The owner elected euthanasia due to poor prognosis. The carcass was submitted for necropsy examination.
Gross Description:
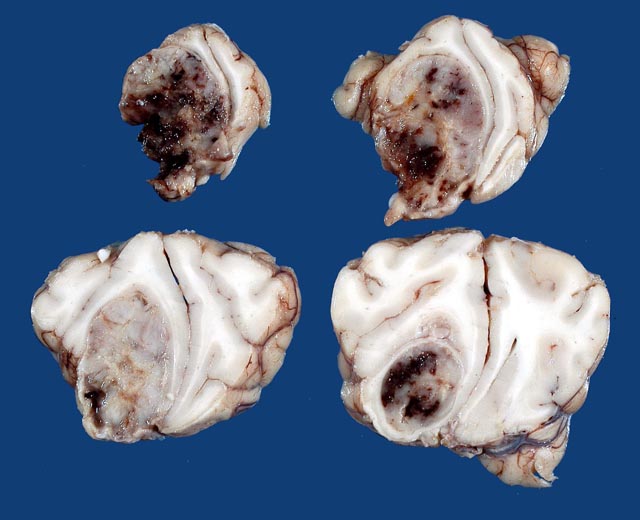
The left cerebral hemisphere showed moderate edematous swelling with flattening of the cortical gyri and narrowing of the sulci. In transverse section there was a mass primarily located in the subcortical white matter of the frontal lobe, which extended cranially to the olfactory bulb and caudally to the pellucid septum. The growth was infiltrative, poorly circumscribed, soft, gelatinous and grayish white, with extensive areas of hemorrhage. The mass compressed surrounding tissues and distorted the cerebral hemispheres (
Fig. 2-1).
Histopathologic Description:
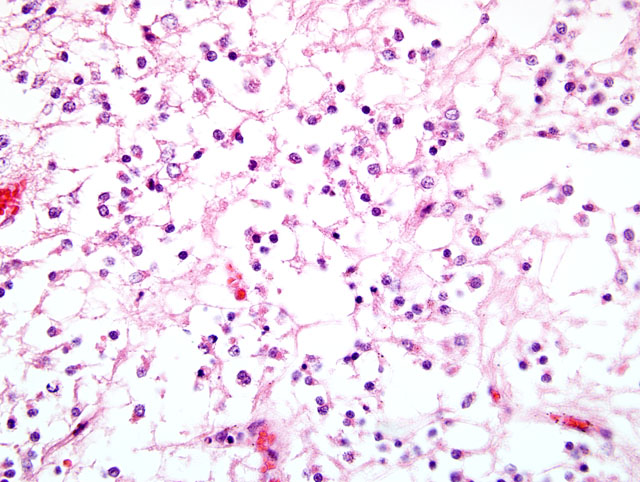
Sections of the brain show a poorly circumscribed, hypercellular mass compressing the surrounding tissue. The tumor is primarily located in the white matter and is composed of dense sheets of small round cells. These cells have moderate amount of clear eosinophilic and vacuolated cytoplasm, with poorly defined cell boundaries(
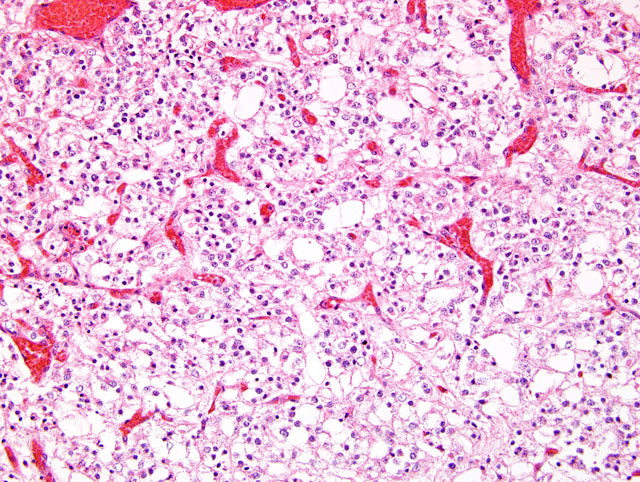
Fig. 2-2). Nuclei are round to oval, hyperchromatic, with clumped chromatin, inconspicuous nucleoli, and show moderate anisokaryosis. Mitotic figures are present (0-3 per random 40x field). Rarely, atypical astrocytes are scattered among the neoplastic cells. Occasionally, in some areas, tumor cells show poorly stained cytoplasm (perinuclear halo) with distinct cell membrane. In other areas, cellularity is low to moderate, and tumor cells have euchromatic nuclei with a vesicular chromatin pattern, single small basophilic nucleoli, fibrillary processes, and microcystic degeneration. The neoplasm exhibits pronounced branching vascular proliferation(
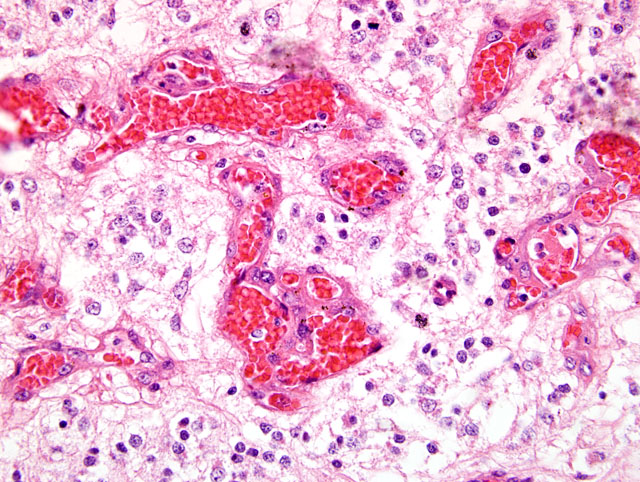
Fig. 2-3) with abundant glomeruloid vessels(
Fig. 2-4). Multifocal extensive areas of necrosis, with hemorrhages and small numbers of foamy macrophages (gitter cells) are also seen. In addition, small spaces filled with pale, gray-blue mucinous material (mucinous cystic degeneration) are also present in some sections (slides not submitted).
Morphologic Diagnosis:
Cerebrum: Oligodendroglioma, anaplastic
Condition:
Oligodendroglioma, high grade
Contributor Comment:
Oligodendrogliomas appear to be the most common primary brain tumors in mature dogs (older than 5 years). Their overall incidence in dogs ranges from 5 to 12 %.(1) A predilection for brachycephalic dog breeds, such as the present case, has been recognized in glial tumors.(2,3) Oligodendroglioma is usually located in the subcortical white matter of the cerebral hemispheres, particularly the frontal lobe. In brachycephalic dog breeds, these tumors appear to arise within remnants of the germinal matrix adjacent to the lateral ventricles. Clinically, the dogs show depression, blindness, ataxia, and change in temperament from nerve damage.(3) Microscopically, the tumor cells have small, round, hyperchromatic nuclei with poorly stained cytoplasm. A perinuclear halo effect is common with delayed fixation which results in the artifactual but characteristically described honeycomb cell pattern.(1) Other important characteristics include pronounced branching capillary proliferation forming a delicate vascular chicken-wire pattern (2) and the formation of vascular loops or glomerular-like tufts often arranged in long lines or clusters, at the margins of or throughout the tumor.(1) Mucinous cystic degeneration and multifocal mineralization sometimes occur.(1,3) Other glial cells such as astrocytes and transitional forms between astrocytes and oligodendrocytes may be present in varying numbers.(2)
There are no antibodies for specific markers for oligodendrogliomas that have been formalin fixed and paraffin embedded.(1) The results of immunohistochemical staining of canine oligodendroglioma with antibody against myelin basic protein are generally negative. Many oligodendrogliomas show a mixture with GFAP-positive astroglial cells. The interpretation of the finding is controversial whether the astrocytes indicate a capacity for immature oligodendrocytes to express astrocytic differentiation or reactive astrocytes.(3)
In our case, the neoplasm was considered to be anaplastic due to the findings of high cellularity in some areas, frequent mitotic figures, nuclear pleomorphism, prominent proliferation of glomeruloid vascular tufts, and extensive necrosis. Anaplastic (malignant) oligodendroglioma and astrocytoma share several histomorphological features, including high cellularity, necrosis, high mitotic rate, and prominent proliferation of glomeruloid vessels; thus, distinguishing between these two glial neoplasms may be difficult.(2) In addition, the presence of intermingled astrocytic cells is common in anaplastic oligodendrogliomas and may further complicate the histologic picture. However, the presence of several distinguishing features in the tumor cells such as round, hyperchromatic nuclei surrounded by small amounts of clear to lightly stained cytoplasm, and distinct cell borders, as well as the branching capillary proliferation, support the diagnosis of oligodendroglioma in this case.
JPC Diagnosis:
Cerebrum: Oligodendroglioma, anaplastic
Conference Comment:
Oligodendrogliomas have been reported in several animal species including dogs, cats, horses, and cattle. Grossly, these tumors are generally well demarcated, gray, grey-pink or pink -red, soft, gelatinous and may have cystic foci.(3,5) Necrosis and hemorrhage are uncommonly present.
The differential diagnosis discussed during the conference included oligoastrocytoma and neurocytoma.Â
Oligoastrocytomas (mixed gliomas) are glial neoplasms with astrocytes and oligodendroglial cells either intermingled or separated into distinct groups of cells.(4) When the cell populations are distinct, the diagnosis is uncomplicated. When the two cell populations are intermixed, at least 25% of the cells must be astrocytes to be classified as an oligoastrocytoma.(4) Tumors with less than this threshold of astrocytes are considered anaplastic oligodendrogliomas with reactive astrocytes.(4) Interpretation of the histogenesis of intratumoral cells can be complicated by the presence of minigemistocytes (gliofibrillary oligodendrocytes) which are neoplastic cells with round, oligodendroglial-type nuclei and GFAP-positive, eccentric eosinophilic cytoplasm.
The first reported case of a neurocytoma in domestic animals involved the spinal cord of a dog and was published in 2008.(2) Neurocytomas stain strongly positive for synaptophysin allowing differentiation from oligodendrogliomas.(3)
References:
1. Burger PC, Scheithauer BW: Tumors of neuroglia and choroid plexus.Â
In: AFIP Atlas of Tumor Pathology, Series 4, Tumors of the Central Nervous System, ed. Silverberg SG, Sobin LH, Series 4, Fascicle 7, pp. 225-233, ARP Press, Washington, DC, 2007
2. Huisinga M, Henrich M, Frese K, Burkhardt, Kuchelmeister K, Schmidt M, Reinacher M: Extraventricular neurocytoma of the spinal cord in a dog. Vet Pathol
45:63-66, 2008
3. Koestner A, Higgins RJ: Tumors of the nervous system.Â
In: Tumors in Domestic Animals, ed. Meuten DJ, 4th ed., pp. 703-706. Iowa State Press, Ames, Iowa, 2002
4. Koestner A, Bilzer T, Fatzer R, Schulman FY, Summers, BA, Van Winkle TJ: Histological classification of tumors of the nervous system of domestic animals.Â
In: World Health Organization Histological Classification of Tumors of Domestic Animals, ed. Schulman FY, 2nd series, vol. 5, pp. 18-21, Armed Forces Institute of Pathology, Washington DC, 1999
5. Maxie MG, Youssef S: Nervous system.Â
In: Jubb, Kennedy and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol 1, pp. 446-448. Elsevier Limited, Philadelphia, PA, 2007
6. Summers BA, Cummings JF, de Lahunta A: Tumors of the central nervous system.Â
In: Veterinary Neuropathology, Summers BA, Cummings JF, de Lahunta A, eds., pp. 370-373, Mosby, St. Louis, MO, 1995