Signalment:
Gross Description:
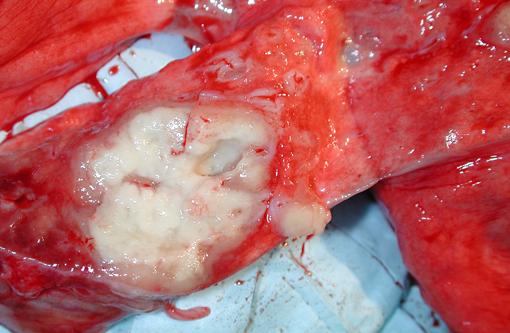
Clusters of enlarged, plump-appearing thoracic lymph nodes were present, especially in the left and right subclavicular and superior paratracheal regions. On transection, these showed partial effacement of normal cortical-medullary nodal architecture with cream-colored homogeneous tissue. However, no definitively recognizable granuloma structures were observable within them. Approximately 75% of the liver effaced by multifocal and coalescing expansile creamcolored solid but soft nodules. These ranged in size from several millimeters to several centimeters (coalescences). The spleen was enlarged to approximately 4 to 5 times its normal size. Visible centrally within the body was a 3 to 4 cm cream-colored soft mass. This lesion was mottled and soft centrally, suggesting early avascular necrosis. Other smaller solid cream colored masses were present within the splenic parenchyma. The right adrenal gland was massively enlarged (3 to 4 cm) and totally effaced by a similar appearing homogeneous cream colored mass and a 2 cm mass was present in the caudal pole of the right kidney. A large portion of one of the poles of the left adrenal gland was also replaced by a solid, grey-colored mass. Mesenteric lymph nodes were markedly enlarged, homogeneous and without normal-appearing central architecture. The GI tract was considered unremarkable grossly. No lesions were noted in the remainder of the abdominal viscera. Inguinal and axillary lymph nodes were enlarged 2-3 times normal size and had an appearance on cut surface similar to that described for the thoracic lymph nodes examined.
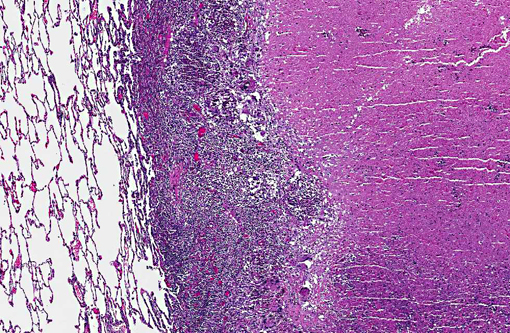
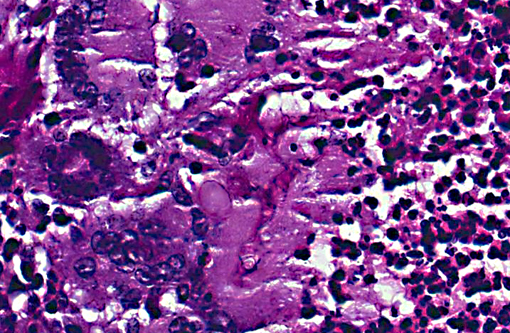
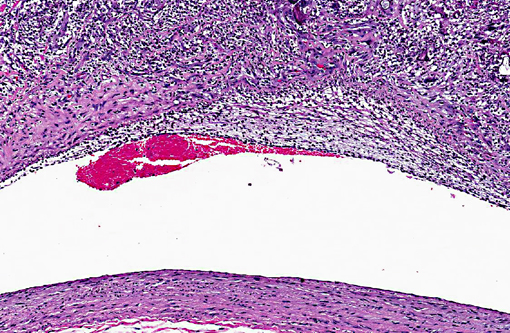
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Pulmonary cavities are frequent manifestations of a wide variety of inflammatory processes involving the lung and considerable variation exists in the pathophysiology of their development. The term itself (cavity) has somewhat different pathological and radiological definitions, generally connoting air/gas filled spaces with definable walls of various thicknesses.(9) Other related and overlapping terms include lung abscess (a necrotizing lung infection characterized by pus or other inflammatory material filled cavity) and pulmonary mycetoma. The latter somewhat dated term is occasionally used to reference a fungal ball within a pre-existing lung cavity, although the designation mycetoma more properly refers to a chronic subcutaneous infection caused by actinomyces or fungus. In humans the list of conditions leading to cavitation is extensive.(3) Non-infectious entities potentially resulting in lung cavities include malignancies, rheumatologic diseases (especially Wegeners granulomatosis) and pulmonary infarction and necrosis. A variety of common bacterial infections (S. pneumonia, H. influenza, Klebsiella pneumoniae, etc) can cause cavitary pneumonias as well as less common agents such as Nocardia, Actinomycosis, Burkholderia (Melioidosis) and Rhodococcus. Mycobacterium, both tuberculous and non-tuberculous, are widely known to lead to cavitary change; in fact, Mycobacterium tuberculosis generally has the highest prevalence of cavities among persons with pulmonary disease of any infection.(3) Of parasitic causes of lung cavitation, Echinococcus and Paragonimus are the most commonly mentioned. Fungal etiologies also abound and include Aspergillus (often arising as fungal balls within a pre-existing cavity), Histoplasma, Blastomyces, Coccidioides, and Pneumocystis.
The fungus causing infection in this animal was identified by culture as Cunninghamella bertholletiae. This saprophytic, filamentous species is found primarily in soil and is one member of the Zygomycetes class of fungi, diseases from which are broadly referred to as zygomycosis.(2) Molds in this class are comprised of two fungal groups of primary medical importance the orders Mucorales and Entomophthorales. Infections caused by Mucorales were formerly termed phycomycosis, a term no longer used.(7) C. bertholletiae is classified under this order and is the only member of its genus proven to be pathogenic. It has been increasingly reported as an emerging pathogen(4) causing disease in a wide range of immunosuppressed human patients with debilitating factors usually related to diabetes mellitus, corticosteroid treatment or granulocytopenia. Interestingly, T-cell dependent immunity does not play an essential role in the defense of this infection and AIDS patients are not particularly susceptible to these organisms.5 Infection with Cunninghamella has rarely been reported in immunocompetent hosts.(10)
Although spontaneous zygomycosis is recognized not uncommonly in a variety of species,(8) naturally occurring Cunninghamella infections are not as widely reported in animals. However, murine models of the disease have been developed.
Whether the monkey in this case had a pre-existing primary cavitary lung lesion of some other pathogenesis (e.g. Trematode parasite, acariasis, etc) which subsequently became colonized after immunosuppression with this zygomycosis or the Cunninghamella arrived in conjunction with a primary cavity forming insult and remained contained until SIV infection is not clear nor will the exact series of events ever be ascertainable. There was no morphological or culture evidence of concurrent infectious or parasitic agents.
JPC Diagnosis:
Conference Comment:
In humans, C. bertholletiae has been shown to exhibit a higher pathogenicity and a poorer prognosis than infections with other members of the order Mucorales, specifically Rhizopus and Mucor species.(6) Neutrophils play an important role in the clearing of C. bertholletiae infection; thus its resistance to neutrophil-induced damage via IL-8 suppression accounts in part for its increased virulence. IL-8 is a potent chemotactic factor and thus its suppression results in a decrease in the number of neutrophils recruited. Other mechanisms of neutrophil suppression, including resistance to iron chelation, fungal mass, and modulation of TNF-alpha related responses, appear to enhance the virulence of this interesting zygomycete.(6)
References:
2. Chayakulkeeree M, Ghannoum MA, Perfect JR. Zygomycosis: the re-emerging fungal infection. Eur J Clin Microbiolo Infect Dis. 2006;25:215-229.
3. Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clinical Microbiol Rev. 2008;21(2):305-333.
4. Honda A, Kamei K, Unno H, Hiroshima K, Kuriyama TA. Murine model of zygomycosis by Cunninhamella bertholletiae. Mycopathologia. 1999;144:141-146.
5. Kamei K. Animal models of zygomycosis Agsidia, Rhizopus, Rhizomucor and Cunninhamella. Mycopathologia. 2000;152:5-13.
6. Gomes MZR, Lewis RE, Kontoyiannis DP. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin Microbiol Rev. 2011;24(2):411445.
7. Migaki G, Schmidt RE, Toft JF, Kaufmann AF. Mycotic infections of the alimentary tract of nonhuman primates: a review. Vet Pathol. 1982;19:93-103.
8. Sondhi J, Gupta P, Sood N. Experimental zygomycosis in rabbits: clinicopathological studies. Mycopathologia. 1999;144:29-37.
9. Tuddenham WJ. Glossary of terms for thoracic radiology: recommendations of the nomenclature committee of the Fleischner society. Am J Roentgenol. 1984;143:509-517.
10. Zeilender S, Drenning D, Glauser FL, Bechard D. Fatal Cunninghamella bertholletia in an immunocompetent patient. Chest 97. 1990;1482-1483.