Signalment:
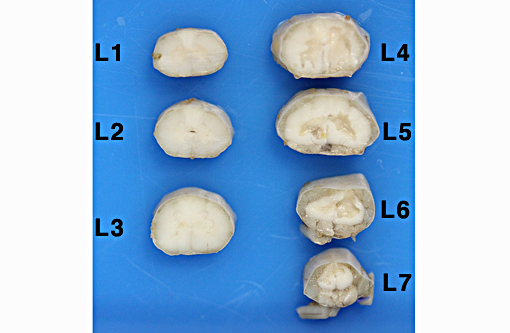
Gross Description:
Histopathologic Description:
Morphologic Diagnosis:
Lumbar spinal cord (L2-L7): Poliomyelomalacia, severe, locally extensive with intralesional fibrocartilaginous emboli, Gitter cell infiltration and cavitation (infarction), consistent with fibrocartilaginous embolic myelopathy (FCEM)
Lumbar spinal cord (L2-L7): Myelin vacuolation, ventral, lateral and dorsal funiculi myelin vacuolation with Gitter cell infiltration, axonal spheroid formation, (Wallerian degeneration) and gliosis with gemistocytic astrocytosis
Lumbar spinal cord (L2-L7):Myelin vacuolation and loss, spinal nerves with Schwann cell proliferation (B+â-+ngners bands, Wallerian degeneration) and neuritis, multifocal lymphoplasmacytic, mild
Lab Results:
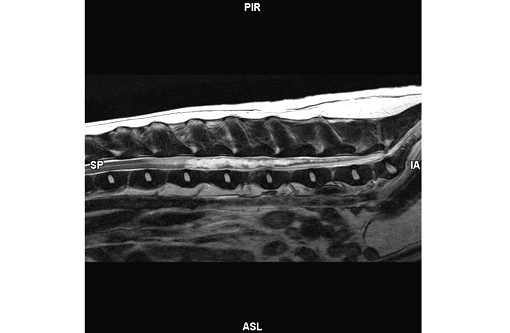
MRI report: Fairly well margined, irreg-ularly shaped and undulating T2 hyperintensity is within the spinal cord from the level of L2-3 caudally to the conus medullaris (T2W, Figure 1). This area remains heterogeneously hyperintense on flair images and is hypointense on T1 weighted images without evidence of contrast enhancement. There is no evidence of signal voids on the FFE sequences. The spinal cord is increased in diameter over this region. The disc spaces are essentially normal with only a minimal amount of narrowing to the nucleus pulposus of 4-5.
Diagnosis: Locally extensive intramedullary myelopathy, from L2 caudally.
Comments: Consideration is given to noncontrast enhancing neoplasia. Another perhaps less likely differential could include intramedullary cyst formation. The persistent hyperintensity on flair images makes a cyst alone unlikely.
Condition:
Contributor Comment:
The embolizing material has been identified as fibrocartilage histologically and is histochemically identical to the nucleus pulposus of the intervertebral disc. There are multiple hypotheses to explain how the fibrocartilaginous material enters the spinal vessels.(3) The pathophysiology of this process is controversial and the exact mechanism is poorly understood.(4)
- Direct penetration of nucleus pulposus fragments into spinal cord or vertebral vessels. Penetration into thin walled veins is considered more likely, however, injection entrance or arteriovenous anastomoses could explain the presence of emboli in arteries. In large breed and non-chondrodystrophoid dogs, the nucleus pulposus remains soft for a longer period of time, which may predispose to mechanical vascular injection with type II disc disease.(2,4,6)
- Chronic inflammatory neovascularization of the degenerated intervertebral disc, with penetration of nucleus pulposus fibrocartilage into the newly formed vessels after a sudden rise of intervertebral disc pressure.(3)
- Remnant embryonic vessels within the nucleus pulposus, which is normally avascular in adults.(3)
- Mechanical herniation into the vertebral bone marrow sinusoidal venous channels with retrograde entrance into the basivertebral vein and internal vertebral venous plexus. In humans, this hypothesis is supported by the presence of Schmorls nodules (masses of fibrocartilage within the vertebral body cancellous bone), however, these are extremely rare in dogs.(3)
- Fibrocartilage may arise from vertebral growth-plate cartilage in immature dogs or metaplasia of the vascular endothelium, which ruptures into the lumen and embolizes within the spinal cord intrinsic vasculature.(3)
Other types of material which can occlude vessels include thrombi, bacterial, parasitic, neoplastic or fat emboli. FCEM should be differentiated from noncompressive nucleus pulposus extrusion, in which nondegenerated nucleus pulposus extrudes during strenuous exercise or trauma, causes a spinal cord contusion and dissipates within the epidural space. Other differential diagnoses are compressive intervertebral disc extrusion, infectious and immune mediated myelitis, neoplasia and intra- and extramedullary hemorrhages.(3)
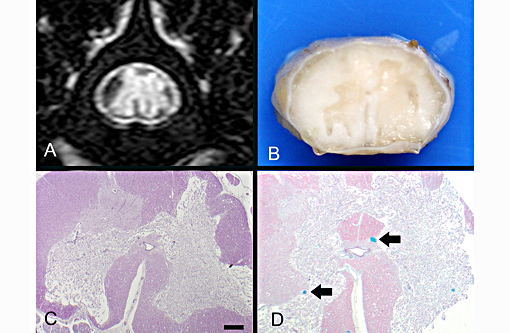
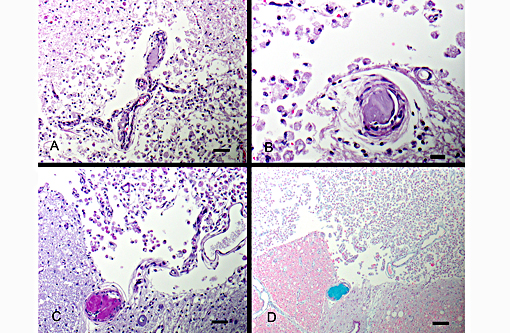
Definitive diagnosis of FCEM can only be obtained with histologic examination of the affected spinal cord segments, which demonstrates fibrocartilaginous material in spinal vessels within or near an area of focal myelomalacia.(3-5) The infarcted region is most severe at the center of the lesion and tends to taper off in adjacent cranial and caudal segments.(5) The distribution reflects the supply of the occluded vessels, and is typically asymmetric. As in this case, grey matter is generally more severely affected than white matter, and lesion margins are typically well delineated.(3,4) At gross examination, the spinal cord is commonly swollen and may appear grey or hemorrhagic.(5) Severely malacic regions can be replaced by large sheets of macrophages embedded within a vascular network,(4) or with cavitation. Infarction within a spinal funiculus will result in focal axonal swelling and spongy ballooning of myelin at its margins. If the ventral horn grey matter is infarcted, Wallerian degeneration develops in its neuronal projections in the ventral funiculus and in the ventral spinal roots.(4,5) Fibrocartilaginous material can be observed in both arteries and veins. In some cases, it can be difficult to identify the type of vessel occluded due to attenuation of the wall by the embolism. Emboli are amphophilic in H&E sections, magenta with PAS (Figure 4C) and blue with Alcian blue stains (Figures 3D, 4D). Attempts at organization are manifest as endothelial coverage, also observed in these sections.(5)
In order to produce infarction of the spinal cord parenchyma, multiple intramedullary tributaries must be occluded.(5) The ventral spinal artery (VSA) extends along the ventral median fissure for the entire length of the spinal cord, and is the largest in diameter in the cervical and lumbar regions.(3) The VSA gives rise to the central arteries, which supply most of the grey matter and part of the lateral and ventral white matter of the spinal cord, with asymmetric distribution at several spinal cord segments.(3) The dorsal spinal arteries are also continuous throughout the spinal cord, have the largest diameter in the cervical and lumbar regions and supply the dorsal white and grey matter. The anastomotic plexus on the surface of the spinal cord gives rise to radial arteries that enter the spinal cord and supply the lateral and ventral white matter.(3) In our case, the central arteries were most frequently occluded vessels, resulting in severe ischemic necrosis of predominantly the grey matter.
Prognosis is dependent upon severity and extent of the ischemic injury. One study found that 84% of dogs had either complete clinical recovery or partial recovery with return to normal function as a pet. The lesion length-vertebral length ratio has been associated with outcome. This is the ratio between the length of the intramedullary hyperintensity on mid-sagittal T2 weighed images and the length of the vertebral body (C6 in dogs with cervical lesions or L2 in dogs with thoracolumbar lesions). Dogs with a lesion-length vertebral length ratio (LL:VL) greater than 2.0 or a percent cross sectional area of the lesion of 67% or greater were significantly more likely to have an unsuccessful outcome than those with lower values.(3) In this case, the LL:VL was 7 (11.12/1.6) and the percent cross sectional area of the lesion was 75%. Lower motor neuron signs have been correlated with a poor prognosis, but distinction between upper and lower motor neuron signs was not found to be statistically significant in one case series, in which extension of the lesion appeared to be a more important prognostic factor than localization.(4) Absence of deep pain perception was significantly associated with dogs that were later euthanized, and was determined by some authors to be the most important negative prognostic factor observed.(4) This change is likely associated with severe, bilateral grey and white matter damage. Additional negative prognostic factors were involvement of intumescences and symmetrical clinical signs. Immediate physiotherapy and hydrotherapy had a major positive influence on the recovery rate.(4)
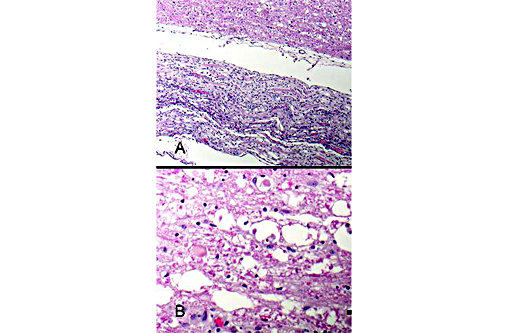
Figure 5: Spinal nerves are hypercellular with myelin loss and Schwann cell proliferation (B+â-+ngners bands), (100x, bar is 100um, A). The spinal cord surrounding the infarcted regions undergoes myelin vacuolation with digestion chamber formation, Gitter cell infiltration, gliosis and axonal swelling (spheroids, Wallerian degeneration), (400x, bar is 500um, B).
JPC Diagnosis:
Conference Comment:
The contributor provides an excellent review of FCEM in dogs. In addition to being reported in dogs, FCEM (though much less common) has also been reported in other species including horses, pigs, turkeys and cats. Of note, FCEM usually occurs in middle aged to older cats, with equal representation between males and females, and most commonly occurring at the C6-T2 spinal cord segments.(2) Interestingly, Horners syndrome has also been associated with FCEM in cats when the lesion is localized to the T1-T3 spinal cord segments.(1)
References:
1. Barker EN, Schofield E, Granger NP. What is your Neurologic Diagnosis? J Am Vet Med Assoc. 2014;245(1):49-51.
2. Cauzinille L, Kornegay JN. Fibrocartilaginous embolism of the spinal cord in dogs: review of 36 histologically confirmed cases and retrospective study of 26 suspected cases. J Vet Intern Med. 1996;10(4):241-245.
3. DeRisio L, Platt SR. Fibrocartilaginous embolic myelopathy in small animals. Vet Clin Small Anim. 2010;40:859-869.
4. Gandini G, Cizinauskas S, Lang K, Fatzer R, Jaggy A. Fibrocartilaginous embolism in 75 dogs: clinical findings and factors influencing the recovery rate. J Sm Anim Prac. 2003;44:76-80.
5. Summers BA, Cummings JF, de Lahunta A. Degenerative diseases of the central nervous system. In: Veterinary Neuropathology. St. Louis, MO: Mosby; 1995:246-249.
6. Thompson K. Bones and Joints. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 1. Philadelphia, PA: Elsevier Saunders; 2007:156-157.
Zachary JF. Cardiovascular System and Lymphatic Vessels. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed., St. Louis, MO: Mosby Elsevier; 2012:585-586.