CASE 4: A19-1839 (4152506-00)
Signalment:
17 years, female, military macaw, Ara militaris, macaw
History:
Regurgitation and wasting.
Gross Pathology:
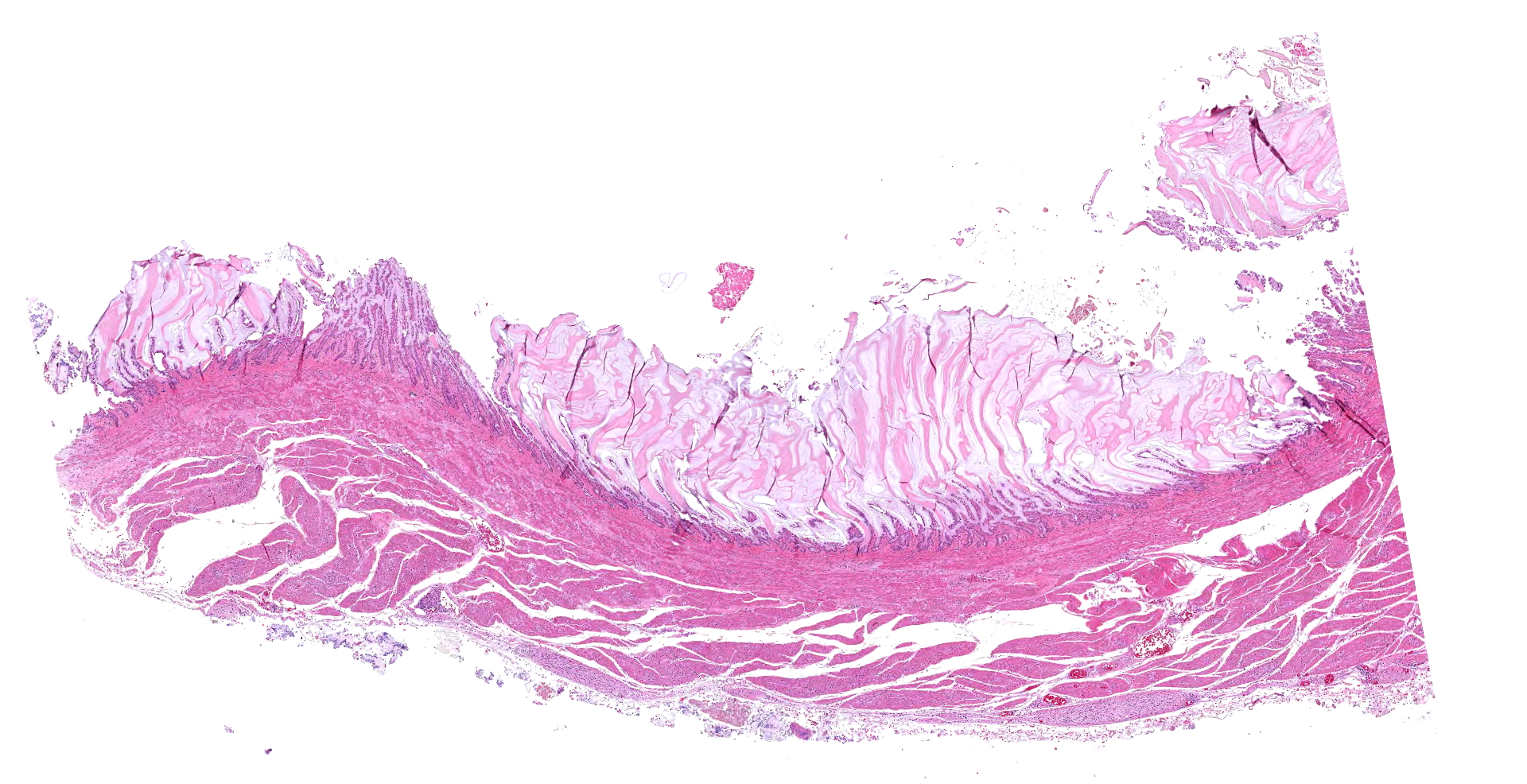
The 780 g macaw was wasted with severe pectoral muscle atrophy and no appreciable coelomic adipose tissue. The proventriculus (not submitted to WSC) was distended with feed and approximately 8 cm in length. The wall of the proventriculus was markedly thin. The proventriculus was adhered to nearby tissues by strands of fibrin.
The ventriculus (submitted tissue) was flaccid with severe, diffuse thinning of the muscular wall.
Laboratory results:
Aerobic culture of the liver and culture of liver and gastrointestinal tract for Salmonella were negative.
No parasites were observed on fecal flotation.
Microscopic description:
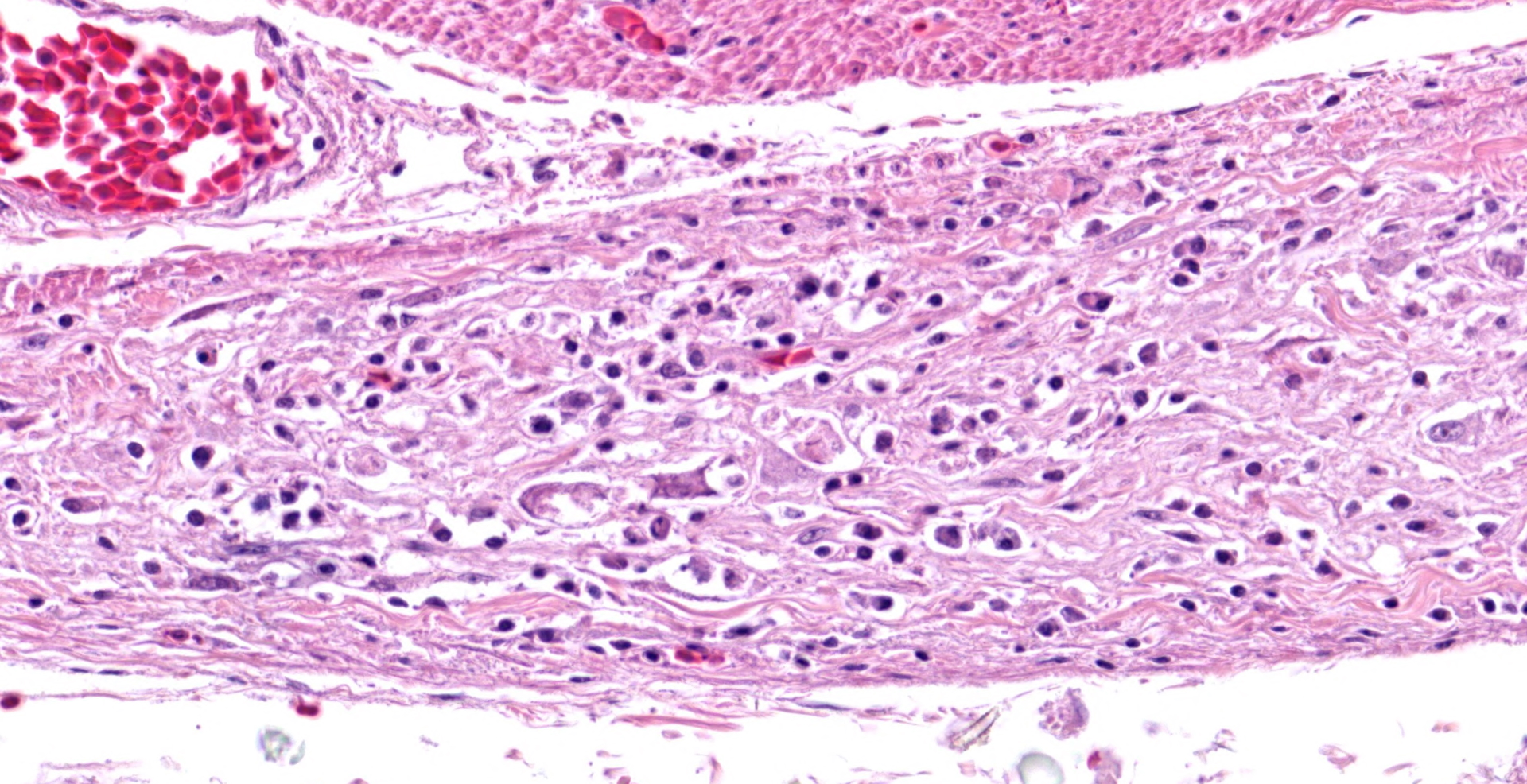
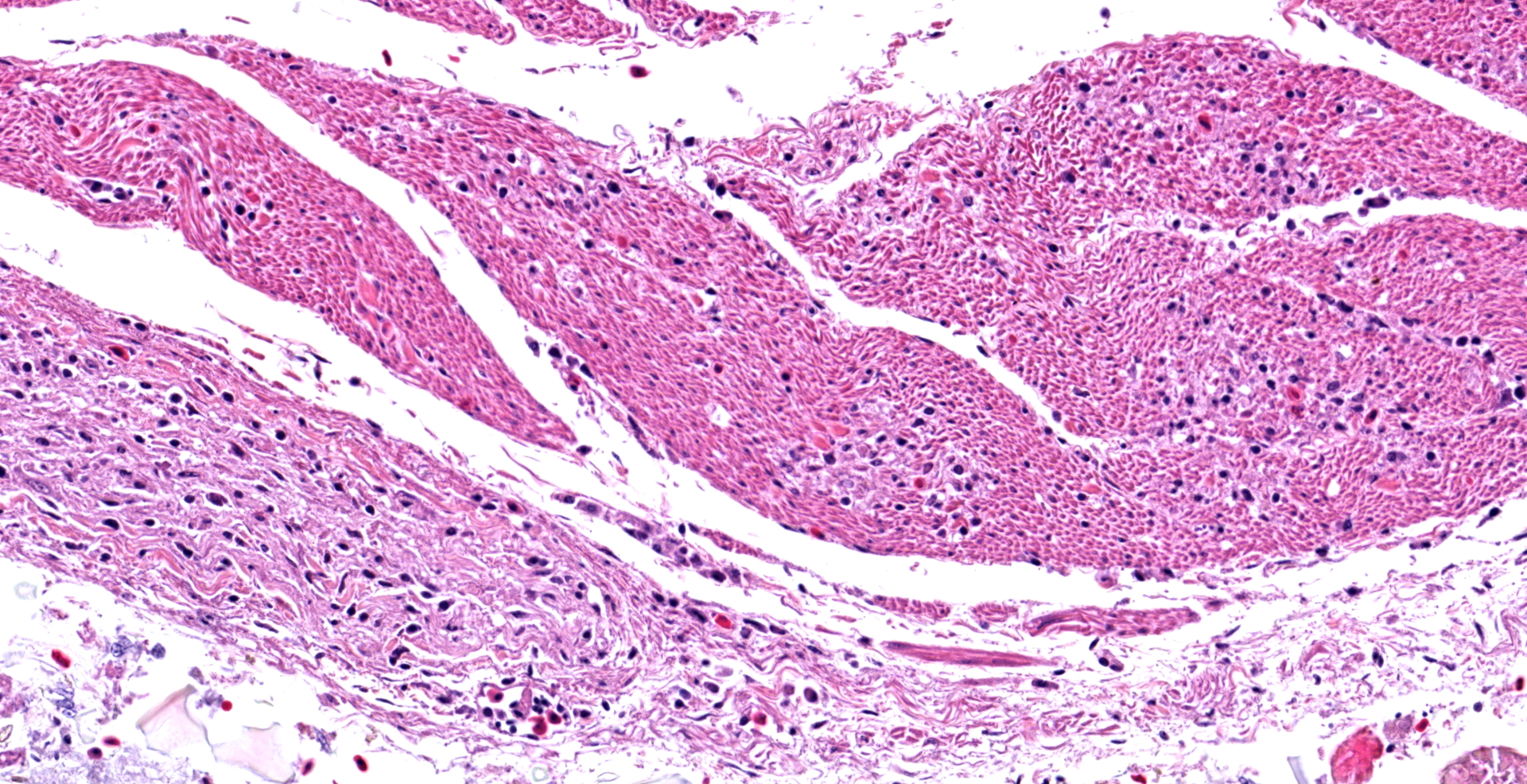
In the section of ventriculus, numerous lymphocytes and plasma cells are in nerve fiber bundles and ganglia of the autonomic plexi. Smooth muscle bundles of the tunica muscularis are atrophied with variable lymphocytic inflammation. Axons and neuronal cell bodies are degenerated or lost with a relative increase in endoneurial fibrous tissue. Similar histologic lesions were in the crop and proventriculus (not submitted).
Contributor's morphologic diagnosis:
Lymphoplasmacytic ganglioneuritis with muscular atrophy, ventriculus
Contributor's comment:
The lymphoplasmacytic inflammation of gastrointestinal autonomic plexi prompted a diagnosis of proventricular dilatation disease (PDD). Coupled with the gross lesions of proventricular dilation, the autonomic ganglioneuritis is considered diagnostic of PDD. Immunohistochemistry and PCR can be used for confirmation, using brain, especially thalamus or hind brain, or other tissues.8 Dilation of the proventriculus (distension by feed), the major gross lesion at autopsy, is attributed to the ganglioneuritis in the autonomic nervous system, especially that of the gastrointestinal tract.8 Other lesions can include encephalomyelitis, peripheral neuritis, myocarditis, and adrenalitis.
A viral cause had long been suspected for PDD, and in 2008, separate research teams identified novel avian bornaviral strains by DNA sequencing and PCR of the brain and other tissues from parrots with PDD.3,5 Originally named avian bornavirus, the etiologic agent is now called parrot bornavirus. Fecal-oral transmission has been proposed as the natural route of infection; however, oculonasal or oral inoculation failed to establish persistent infection or disease in cockatiels.2 Intramuscular (pectoral) inoculation with parrot bornavirus-2 consistently resulted in infection, with virus appearing first in the caudal segments of the spinal cord with rostral spread to the brain.6 The gastrointestinal autonomic nervous system was not evaluated in that study, but in natural infection, gastrointestinal signs, attributable to ganglioneuritis of the autonomic nervous system, typically precede the development of signs of encephalomyelitis.
Clinical signs of PDD3 include regurgitation and weight loss, which were reported in this macaw. Infected birds can carry the bornavirus asymptomatically and shed it intermittently in feces and urates. Clinical signs develop as the bird mounts an immune response to the virus. The lymphoplasmacytic inflammation causes progressive destruction of peripheral nerves, ganglia, spinal cord, and brain. Disease progression can be slow or rapid.
Contributing Institution:
Purdue University
Animal Disease Diagnostic Laboratory: http://www.addl.purdue.edu/
Department of Comparative Pathobiology: https://vet.purdue.edu/cpb/
JPC diagnosis:
Ventriculus: Ganglioneuritis and leiomyositis, lymphoplasmacytic, multifocal, moderate, with neuronal necrosis and myofiber atrophy.
JPC comment:
Currently, there are 15 enumerated avian bornavirus (ABV) genotypes that make up six viral species, which include psittaciform bornavirus (PaBV) 1, 2, and 47, passeriform bornavirus-1, passeriform bornaavirus-2, and waterbird bornavirus 1. Within these species are often several strains specific to different host bird species. Shedding of the virus is intermittent, but PCR testing of urine, feces, and cloacal swaps are most likely to contain ABV RNA. However, a negative result does not rule out infection.4
There are a number of other conditions that may result in similar clinical signs as ABV, some of which include gastrointestinal parasites, tumors, papillomas, granulomas, foreign bodies, or heavy metal toxicity (particularly lead).4
If available, immunohistochemistry (IHC) is a useful modality to evaluate tissue for ABV infection. Anti-ABV antigen is most often located in the nucleus of affected cells, such as ganglia, brain, spinal cord, adrenal glands, pancreas, kidneys, anterior gastrointestinal tract, heart, testes, ovaries, and thyroid glands.3,7 Investigation into lymphoplasmacytic infiltration of Purkinje fibers, epicardium, myocardium, endocardium, and great vessels of naturally and experimentally infected birds with PaBV was closely associated with epicardial ganglioneuritis. In many cases, affected cells were immunolabeled with anti-PaBV antibodies.1,7
Avian bornaviruses share characteristics with their mammalian counterparts, including a well-established correlation between the onset of neurologic signs and the presence of CD3+ T lymphocytes in the brain. Beyond the lesions noted in the central nervous system, inflammatory infiltrates are most often composed of lymphocytes, macrophages, and variable numbers of plasma cells, suggesting an important link between clinical disease and the adaptive immune system. Avian bornavirus also appears to inhibit type 1 IFN signaling, as well as trim viral genomic 5'-phosphates to evade recognition by RIG-1 receptors. Current antiviral therapies are working to leverage these mechanisms.7
During case discussion, one pathologist experienced with avian pathology opined that the inflammation in smooth muscle in this case may not represent true leiomyositis per se, but inflammation extending along neuronal axons as they traverse the adjacent smooth muscle.
References:
- de Araujo JL, Hameed SS, Tizard I, et al. Cardiac lesions of natural and experimental infection by parrot bornaviruses. J Comp Path. 2020;174:104-112.
- Heckmann J, Enderlein D, Piepenbring AK, et al. Investigation of different infection routes of parrot bornavirus in cockatiels. Avian Dis. 2017;61:90-95.
- Honkavuori KS, Shivaprasad HL, Williams BL, et al. Novel borna virus in psittacine birds with proventricular dilatation disease. Emerg Infect Dis. 2008;14(12):1883-1886.
- Hoppes SM, Shivaprasa HL. Update on Avian Bornavirus and Proventricular Dilitation Disease. Vet Clin Exot Anim. 2020;23:337-351.
- Kistler AL, Gancz A, Clubb S, et al. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: identification of a candidate etiologic agent. Virol J. 2008;5:88-102.
- Leal de Araujo J, Rodrigues-Hoffmann A, Giaretta PR, et al. Distribution of viral antigen and inflammatory lesions in the nervous system of cockatiels (Nymphicus hollandicus) experimentally infected with parrot bornavirus 2. Vet Pathol. 2019;56(1):106-117.
- Nobach D, Muller J, Tappe D, Herden C. Update on immunopathology of bornavirus infections in humans and animals. Advances in Virus Research. 2020;107:159-222.
- Venâncio Donatti R, Resende M, Ferreira Junior FC, et al. Fatal proventricular dilatation disease in captive native psittacines in Brazil. Avian Dis. 2014;58:187-193.