Signalment:
Gross Description:
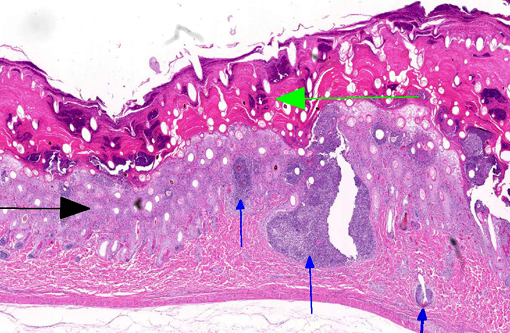
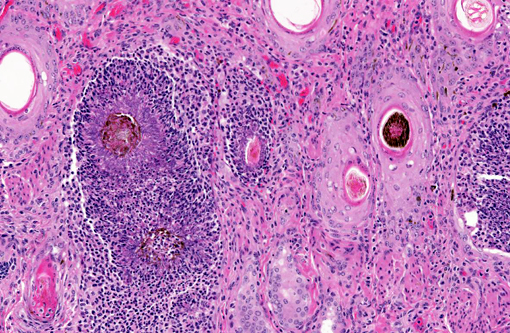
Histopathologic Description:
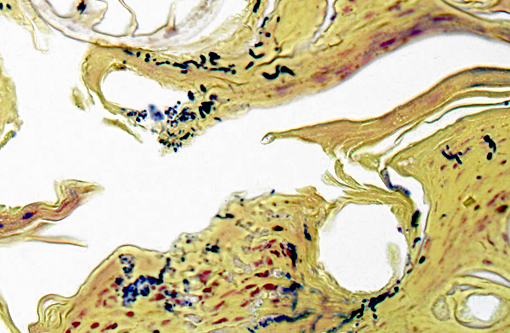
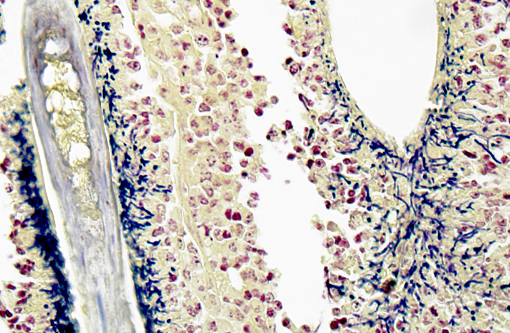
Gram Stain (not submitted): The filamentous and coccoid forms of bacteria stain gram-positive.
Morphologic Diagnosis:
Condition:
Contributor Comment:
The first reports in white-tailed deer were from the New York state region in the 1970s, along with an isolated case in a fawn from South Carolina.(3,5) A recent survey of bacterial and parasitic dermatologic diseases in white-tailed deer from 1975-2012 found a 0.7% incidence (19/2569) of dermatophilosis.(4) At 21.6% of the cases, this was second only to demodicosis in frequency of bacterial or parasitic skin disease among deer in the Southeastern United States.(4) As in initial reports in twin fawns,(5) lesions most commonly involved the head/face and limbs and consisted of exudation and crusting, alopecia, ulcers and erosions.(4) In the current case, the lesions were particularly severe on the face but were also generalized. It has been hypothesized that a predilection for the rostrum in fawns could be due to transmission from an infected dam during nursing.(5) Dermatophilosis has been found in fawns as young as 2 weeks old,(5) and the study by Nemeth et al. found significantly more juveniles affected (63%) than adults.(4) Debilitation and emaciation in the fawn in this case were attributed to the severity of the dermatophilosis, which has been reported as a cause of death in other affected fawns.(3,4)
The diagnosis of dermatophilosis in this case was based on the presence of thick lamellated crusts in association with characteristic filaments of parallel rows of Gram-positive coccoid bodies (railroad track configuration) formed from transverse and longitudinal septation.(2) A combination of skin damage and prolonged moisture are generally required for establishment and spread of infection, as the organism is minimally invasive in intact epidermis, and wet conditions are needed for activation of coccoid bodies into motile zoospores.(2) Ectoparasites can be a cause of trauma and may also act as mechanical vectors to spread the infection.(2) Although usually confined to the epidermis and outer root sheaths of hair follicles, there are rare reports of deeper skin or lymph node involvement.(1,2) Ulceration and furunculosis were prominent features in the current case, but there was no evidence of spread to lymph nodes or other tissues.Â
JPC Diagnosis:
Conference Comment:
Dermatophilus produces exoenzymes and has an affinity for a low carbon dioxide environment which facilitates its colonization and tissue-specificity for the epidermis. The host responds to infection with cornification of keratinocytes and invasion of neutrophils effectively walling off the bacteria from the dermis. The progenitor cells of the follicular epithelium then propagate, forming a new layer of epidermis.(2) Subsequent invasion of this new layer followed by repetitive and unsuccessful defense responses of the host creates the thick laminar and parakeratotic crusts with entrapped hair follicles which are so characteristic to this bacterial infection and nicely exemplified in this case.Â
References:
1. Byrne BA, Rand CL, McElliott VR, Samitz EM, Brault SA. Atypical Dermatophilus congolensis infection in a three-year-old pony. J Vet Diagn Invest. 2010;22:141-143.
2. Ginn PE, Mansell JEKL, Rakich PM. Skin and appendages. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Vol. 1. 5th ed. Edinburgh, UK: Elsevier Limited; 2007:681-682.
3. Gordon MA, Salkin IF, Stone WB. Dermatophilus dermatitis enzootic in deer in New York state and vicinity. J Wildl Dis. 1977;13(2):184-190.Â
4. Nemeth NM, Ruder MG, Gerhold RW, et al. Demodectic mange, dermatophilosis, and other parasitic and bacterial dermatologic diseases in free-ranging white-tailed deer (Odocoileus virginianus) in the United States from 1975 to 2012. Vet Pathol. 2014;51(3):633-640.
5. Roscoe DE, Lund RC, Gordin MA, Salkin IF. Spontaneous dermatophilosis in twin white-tailed deer fawns. J Wildl Dis. 1972;11(3):398-401.