Signalment:
29-year-old, 16.27 kg, female Baboon (
Papio sp.).This baboon was selected for euthanasia due to dark loose stools and senescence.
Gross Description:
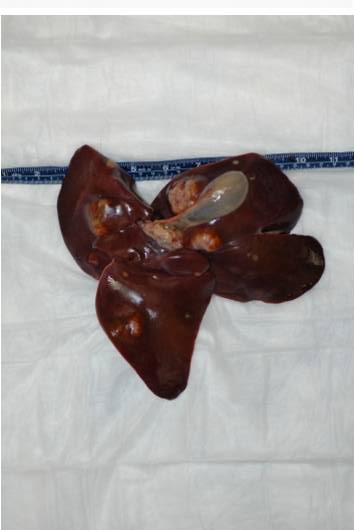
On external examination the animal was found in good body condition and adequately hydrated.
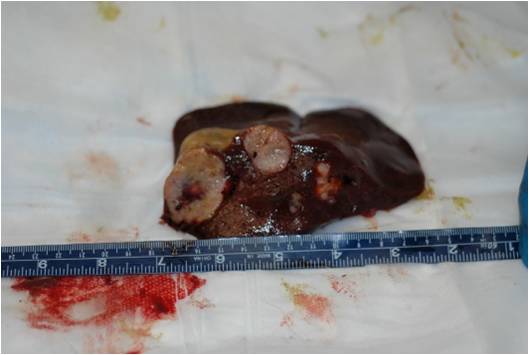
Gross findings included multiple, pale tan to white, irregularly shaped, firm nodules, up to 5 cm in diameter,
multifocally distributed in the hepatic parenchyma.
Histopathologic Description:
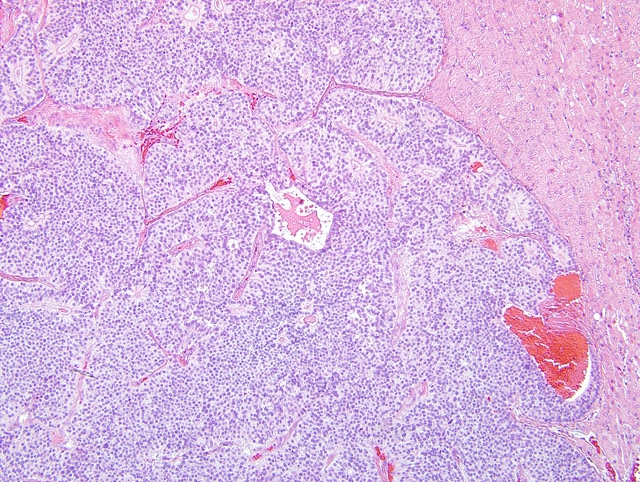
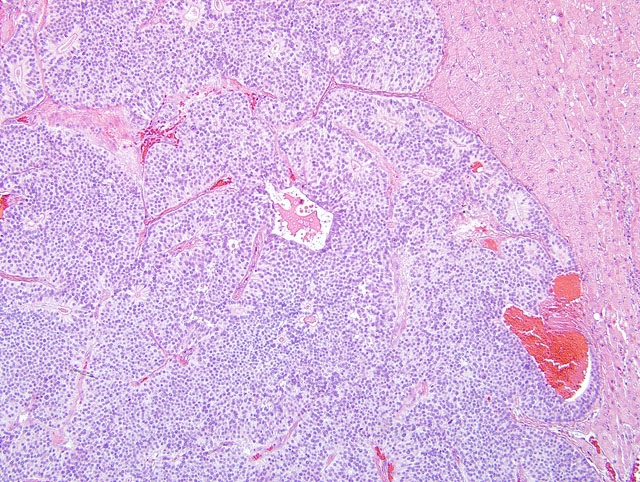
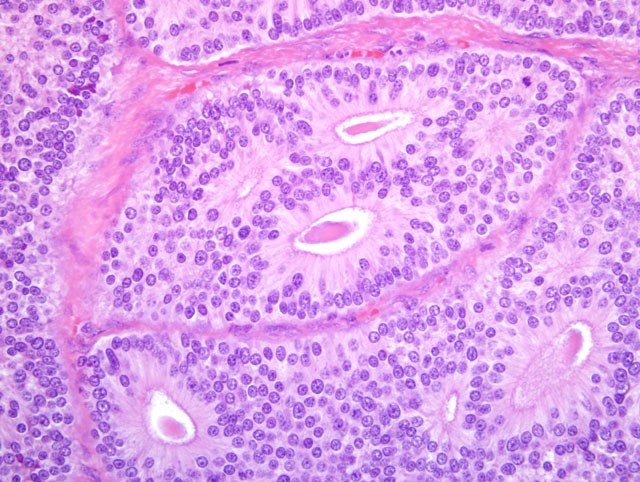
On light microscopy the liver architecture was multifocally effaced and replaced by
a well demarcated, unencapsulated, infiltrative, highly cellular neoplasm composed of nests, packets, and cords of
epithelial cells often forming glandular structures (rosettes and pseudorosettes), supported by a fine to moderate
fibrovascular stroma. The neoplastic cells were cuboidal to columnar with variably distinct cell borders and a
moderate amount of eosinophilic, finely granular cytoplasm. The nuclei were central to basilar, round to oval, with
coarsely stippled chromatin. Mitoses were rare (less than 1 per 10 HPF, 400X magnification). Additional features
included scattered central areas of necrosis and hemorrhage, and variable amounts of homogeneous, eosinophilic
material filling the central lacunae of the rosette formations. The adjacent portions of the non-affected liver
parenchyma showed mild to moderate atrophy from neoplastic compression.
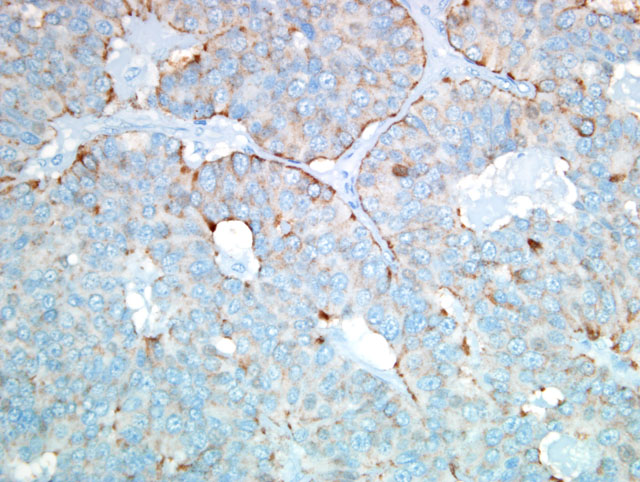
By immunohistochemistry the neoplastic cells were positive for pancytokeratin, chromogranin A, NSE and
synaptophysin and were negative for vimentin, S100 protein, glucagon and insulin.
Morphologic Diagnosis:
Liver: Carcinoma, neuroendocrine, Baboon,
Papio sp.
Condition:
Neuroendocrine carcinoma
Contributor Comment:
The morphologic features, along with the positive staining for the neuroendocrine
markers described above, led to the classification of this neoplasm as a neuroendocrine carcinoma.(1) No similar
neoplastic changes were observed in all the other tissues examined; therefore the liver was considered as the primary
site of origin of the tumor.(1) This case represents the first known primary neuroendocrine carcinoma of the liver to
be reported in a non-human primate.(1-3) There are rare reports of primary hepatic neuroendocrine carcinomas in
humans and domestic animals in the literature. As in this case, neuroendocrine carcinomas in humans are
consistently immunopositive for chromogranin A, NSE, and synaptophysin.(6) This is contrary to what has been
observed in dogs and cats, where the expression of chromogranin A is inconsistent, and NSE and synaptophysin are
considered better indicators.(6) The oval cell is considered to be the progeny of the hepatic stem cells and is
bipotential in nature, giving rise to both hepatocytes and bile duct cells.(4) These cells are located in the terminal
biliary ductules and canal of Hering, which represent the terminal branches of the biliary tree that connects the
interhepatocytic bile canaliculi with the biliary ducts in the portal tracts. They express markers of both immature
hepatocytes (e.g. α-fetoprotein) and bile ducts cells (e.g. bile duct type cytokeratin).(7) In addition, the hepatic
progenitor cell (HPC) compartment has neuro/neuroendocrine features such as the expression of chromogranin A,
neural cell adhesion molecule (NCAM), neurotrophin 4/5, neurotrophin receptor tyrosine kinase B and parathyroid
hormone related peptide.(4) Roskams et al. showed that during the early stages of regeneration, bile duct epithelium
displays neuroendocrine features including cytoplasmic, dense core neurosecretory granules and chromogranin-A
expression while reactive bile ductules have been shown to express NSE.(7)
JPC Diagnosis:
Liver: Neuroendocrine carcinoma, low grade (carcinoid).
Conference Comment:
The contributor has provided an excellent example, accompanied by an informative
overview, of a rare entity. Conference participants contemplated a diagnosis of adenocarcinoma based on the
formation of cystic and tubular structures by neoplastic cells, but uniformly favored the contributors diagnosis
because of the prominent rosette-like glandular structures that have basally-situated nuclei and finely granular
cytoplasm, characteristic of neuroendocrine carcinoma. Because of potential prognostic implications, correctly
distinguishing hepatic neuroendocrine carcinoma from biliary adenocarcinoma or hepatocellular carcinoma is of
paramount importance. For instance, in one study, dogs with hepatic neuroendocrine carcinoma experienced a
significantly shorter survival/euthanasia interval than did those with biliary adenocarcinoma or hepatocellular
carcinoma. In dogs and cats, cytokeratin AE1/AE3 immunostaining is reportedly positive in adenocarcinoma and
negative in hepatic neuroendocrine carcinoma, testifying to the utility of immunohistochemistry in making the
distinction.(6) In the present case, results of the immunohistochemical stains listed by the contributor and repeated
at the AFIP confirmed the diagnosis of neuroendocrine carcinoma.
Conference attendees briefly reviewed the term carcinoid, which is well-ensconced in the texts, where it is
sometimes used interchangeably with neuroendocrine carcinoma. Although the term is used less frequently in the
recent literature, it facilitates professional communication, because unlike neuroendocrine carcinoma, it requires
no further modification to connote low-grade malignancy. In humans, carcinoids are most frequently identified in
the gastrointestinal tract, followed by the tracheobronchial tree and lungs. Among gastrointestinal carcinoids, those
of the jejunum and ileum are most common, followed by those of the colorectum and appendix; those of the
stomach, proximal duodenum, and esophagus are least common. Gastrointestinal carcinoids arise from cells that
release peptide and nonpeptide hormones to coordinate gastrointestinal function; these cells, formerly referred to as
amine precursor uptake and decarboxylation (APUD) cells, comprise the diffuse endocrine system of the
gastrointestinal tract. In humans, carcinoid syndrome is a rare clinical manifestation of the neoplasm characterized
by cutaneous flushing, sweating, bronchospasm, abdominal pain, diarrhea, and fibrosis of right-sided heart valves.
The syndrome results from the secretion and systemic release of vasoactive substances by the tumor, and is strongly
associated with metastatic disease.(8) While carcinoid syndrome has not been reported in animals, conference
attendees discussed neuroendocrine tumor-related paraneoplastic syndromes documented in veterinary medicine,
such as Zollinger-Ellison syndrome due to functional gastrin-secreting tumors, and superficial necrolytic dermatitis,
which is sometimes associated with glucagonomas.
This case was reviewed in consultation with the Departments of Hepatic and Soft Tissue Pathology at the AFIP, both
of which concurred with the above diagnosis, while emphasizing the importance of excluding the possibility of
hepatic metastasis from a primary gastrointestinal carcinoid because of the extreme rarity of primary hepatic
neuroendocrine carcinoma.
References:
1. Aloisio F, Dick EJ Jr, Hubbard GB: Primary hepatic neuroendocrine carcinoma in a baboon (
Papio sp.). J Med
Primatol
38:23-26, 2009
2. Cianciolo RE, Butler SD, Eggers JS, Dick EJ, Leland M, de la Garza M, Brasky KM, Cummins LB, Hubbard
GB: Spontaneous neoplasia in the baboon (
Papio spp.). J Med Primatol
36:61-79, 2007
3. Cianciolo RE, Hubbard GB: A review of spontaneous neoplasia in baboons (
Papio spp.). J Med Primatol
34:51-66, 2005
4. Libbrecht L, Roskams T: Hepatic progenitor cells in human liver diseases. Semin Cell Dev Biol
13:389-396,
2002
5. Patnaik AK, Lieberman PH, Erlandson RA, Antonescu C: Hepatobiliary neuroendocrine carcinoma in cats: a
clinicopathologic, immunohistochemical, and ultrastructural study of 17 cases. Vet Pathol 42:331-337, 2005
6. Patnaik AK, Newman SJ, Scase T, Erlandson RA, Antonescu C, Craft D, Bergman PJ: Canine hepatic
neuroendocrine carcinoma: an immunohistochemical and electron microscopic study. Vet Pathol
42:140-146,
2005
7. Roskams T, Cassiman D, De Vos R, Libbrecht L: Neuroregulation of the neuroendocrine compartment of the
liver. Anat Rec A Discov Mol Cell Evol Biol
280:910-23, 2004
8. Turner JR: The gastrointestinal tract.Â
In: Robbins and Cotran Pathologic Basis of Disease, eds. Kumar V, Abbas
AK, Fausto N, Aster JC, 8th ed., pp. 787-789. Saunders Elsevier, Philadelphia, PA, 2010