Signalment:
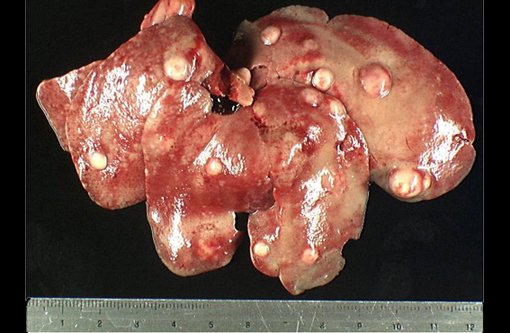
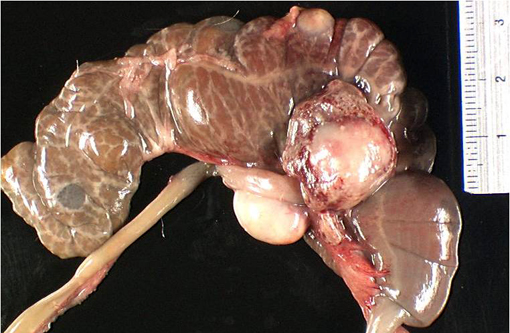
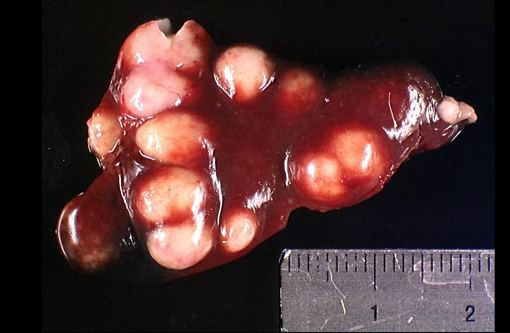
Gross Description:
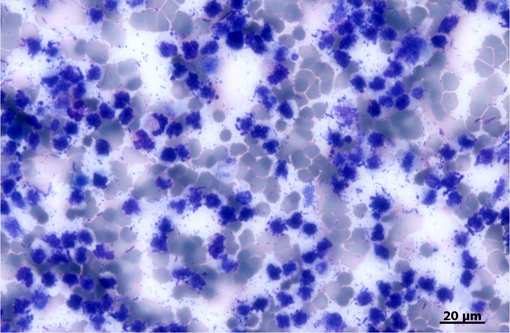
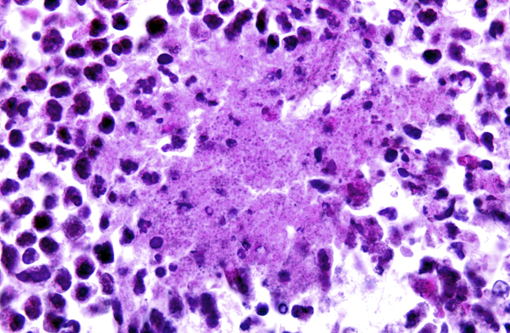
Histopathologic Description:
The largest nodules are 3 to 7 mm in diameter and are made of large colonies of coccobacilli embedded in large amounts of degenerate neutrophils, in turn surrounded by a fibrous and highly vascularized capsule rich in activated macrophages and lymphocytes (multiple hepatic abscesses). Numerous sections of biliary ducts are interspersed in the collagen bundles (biliary duct hyperplasia).Â
The smallest nodules are centered on colonies of coccobacilli admixed with degenerate neutrophils and necrotic, sometimes mineralized hepatocytes surrounded by activated macrophages, plasma cells, and lymphocytes.Â
When visible, portal blood vessels are surrounded by lymphocytes and plasma cells or show invasion of their wall by macrophages and degenerate neutrophils with replacement of their media by a fibrillar eosinophilic material (fibrinoid necrosis).Â
In the remaining parenchyma, the cytoplasm of the hepatocytes is filled with variable-sized clear vacuoles consistent with lipid droplets (micro- and macrovacuolar steatosis).Â
The central veins are congested.Â
The coccobacilli do not stain with either Gram or Ziehl-Nielsen stains.Â
Spleen: The red and the white pulp is disrupted and compressed by multifocal, chronic, 0.5 cm-diameter abscesses and smaller pyogranulomas, with large colonies of intralesional coccobacilli. In the red pulp, siderophages are commonly seen (diffuse hemosiderosis, discrete).Â
Caecum: (autolysis) (absent from the slides)
In the submucosa with extension to the musculosa and the serosa, there is a focal accumulation of degenerate neutrophils and variably mineralized cell debris around large coccobacilli colonies, surrounded by activated macrophages and fibrous tissue. The overlying mucosa is lost (focal ulcer). The remaining mucosa displays a diffuse, discrete infiltration of the lamina propria by lymphocytes and plasma cells.Â
Peritoneum surrounding the caecum: (absent from the slides)
Multifocally, coccobacilli-containing abscesses are centered on blood vessels with fibrinoid necrosis of their wall. Other vessels display only intraluminal thrombi or degeneration of the wall with infiltration by macrophages and degenerate neutrophils.Â
There is an extensive deposit of fibrin and pus in the serosa.Â
Caeco-colic lymph nodes: (absent from the slides)
Two lymph nodes are almost entirely replaced by large abscesses surrounded by a rim of residual lymphoid tissue.Â
There are numerous colonies of coccobacilli in the center of the abscesses. Another lymph node displays numerous macrophages in the subcapsular and medullary sinuses that are seldom binucleated with a large amount of granular cytoplasm (sinusal histiocytosis).Â
Morphologic Diagnosis:
Liver: Hepatitis, suppurative, multifocal, subacute, severe, with intralesional gram-negative coccobacilli colonies consistent with Y. pseudotuberculosis. Steatosis, diffuse, severe.Â
Spleen: Splenitis, suppurative, multifocal, subacute, severe, with intralesional gram-negative coccobacilli colonies consistent with Y. pseudotuberculosis.Â
Caecum: Typhlitis, suppurative and ulcerative, transparietal, subacute, moderate with intralesional gram-negative coccobacilli colonies consistent with Y. pseudotuberculosis. Peritoneum and caeco-colic lymph nodes: Peritonitis and lymphadenitis, suppurative, severe with intralesional gram-negative coccobacilli colonies consistent with Y. pseudotuberculosis.Â
Lab Results:
Condition:
Contributor Comment:
- Salmonella infection (S. typhimurium, S. enteritidis, or S. dublin): there is multifocal granulomatous to suppurative hepatitis, splenitis, lymphadenitis, and enteritis, characterized by paratyphic nodules;
- Tyzzers disease (Clostridium piliforme): there is necrotizing hepatitis, ileitis and typhlitis, often with transmural involvement; the bacteria are intracellular bacilli that react positively with Warthin-Starry stain;
- Streptococcus infection with S. zooepidemicus or S. pneumoniae: these Gram-positive bacteria can be responsible for suppurative lymphadenitis (streptococcal lymphadenitis and pneumococcal infection), hepatitis and splenitis (streptococcal septicemia and pneumococcal infection) (7);
- Mycobacterial granulomas with Ziehl-Nielsen-positive bacteria.Â
Microscopically, the differential diagnosis for large plaque-like colonies of bacteria in H-E sections includes, following the -� YACS -+ mnemonic method:
- Yersinia sp.
- Actinomyces sp., Actinobacillus sp., Arcanobacter sp.,
- Corynebacterium sp., Clostridium sp.Â
- Staphylococcus sp., Streptococcus sp.
The morphology of the bacteria and the results of the Gram staining will further orientate the identification. In the present case, the bacteria were gram-negative cocco-bacilli; these characteristics were consistent with Yersinia sp.Â
Y. pseudotuberculosis and Y. enterocolitica are gram-negative coccobacilli that can survive and grow at low temperature (psychrophilia). The pathogenic strains of theses bacteria share several virulence factors, namely: - the invasin: this adhesin binds to beta-integrins expressed on the surface of the intestinal M cells; it promotes uptake of the bacterium by these cells and triggers the production of chemotactic cytokines by epithelial cells;
- a plasmid-encoded type III secretion system that translocates Yersinia outer proteins (Yops) into the host cell;
- the Yops: these effector proteins allow the bacterium to remain extracellularly and evade phagocytosis and killing by neutrophils and macrophages; one mechanism can be an alteration of the actin cytoskeleton in the host cell as is the case with YopE (4, 8).Â
Y. pseudotuberculosis can also produce a superantigenic toxin termed Y. pseudotuberculosis-derived mitogen a (YPMa). Several pathogenic strains display on their chromosome a high-pathogenicity island (HPI) which carries genes of the yersiniabactin system, involved in siderophore-mediated iron acquisition (5).Â
Infection with Y. pseudotuberculosis is a zoonotic disease; yersiniosis can also occur in various domestic animals and non-human primates. Wild rodents and birds are the reservoir hosts. Transmission is fecal-oral (2, 4, 6). After ingestion, Y. pseudotuberculosis or Y. enterocolitica invade Peyers patches and lymphoid follicles. High numbers of neutrophils are recruited at the portal of entry. The lymphoid follicles and the overlying epithelium are subsequently replaced by suppurative foci. The bacterium then disseminates via lymphatics and hepatic portal veins to the draining lymph nodes and to the liver and the systemic circulation (4), as was the case in this guinea pig.Â
Y. pseudotuberculosis is considered to be an extracellular pathogen. It binds to the macrophages and survives attached to them. The immune response is humoral but also cellular. CD8+ T lymphocytes, considered to protect against intracellular pathogen, have been recently shown to restrict Y. pseudotuberculosis infection. The proposed model is that T cells could target host cells with extracellularly attached Y. pseudotuberculosis, thus allowing the host cells and associated bacteria to be engulfed and removed by neighboring macrophages (3).Â
Yersiniosis was one of the earliest bacterial diseases recognized in guinea pigs. Several types of clinical manifestation are recognized in this species:
- a peracute septicemic form;
- an acute form with miliary, cream-colored nodules in the intestinal wall and ulceration of ileum and caecum;
- chronic infection with caseous nodules in mesenteric lymph nodes, spleen, liver and lung, emaciation, and death;
- non-fatal infection with lesions of lymph nodes of the head and neck. Inapparent carriage in healthy animals is also reported (6, 7).Â
The lesions of Y. pseudotuberculosis and Y. enterocolitica cannot be differentiated either grossly or microscopically. In either case, characteristic microcolonies of coccobacilli are found in microabscesses through histological examination. Confirmation by bacterial isolation is needed (4, 7).Â
Yersinia pestis, the cause of plague in humans and animals, is a clone of Yersinia pseudotuberculosis that emerged 1,500 20,000 years ago (1).Â
JPC Diagnosis:
Conference Comment:
Gut flora is primarily gram-positive bacteria with anaerobic lactobacilli. Coliforms, yeasts, and clostridia may be present in small numbers. A common cause of death in Yersinia infection is the result of the subsequent antibiotic treatment. Certain antibiotic therapies can induce a disruption of normal gut flora which are supplanted by pathogenic bacteria such as Clostridium dificile and Escherichia coli (dysbiosis). Altered microbial ecology in the gut may produce disease and dysfunction because of the intense metabolic activity or antigenicity of the inappropriate bacterial flora to include elaboration of bacterial enzymes which degrade pancreatic enzymes, damage the intestinal brush border, deconjugate and reduce bile acids and alter the intestinal milieu in numerous ways. It is usually the subsequent bacterial antigens and exotoxins that may cause death rather than the initial infection with Yersinia sp (6).Â
Some conference participants interpreted perceived changes in the hepatic arteries as fibrinoid vascular necrosis, noting apparent hypereosinophilia and hyalinization in the vascular walls; however, the moderator believed this to be an artifact due to perceived staining differences, resulting from the use of saffron with standard hematoxylin and eosin..Â
References:
2-AFIP POLA notes, 2007.Â
3- Bergman MA, Loomis WP, Mecsas J, Starnbach MN, Isberg RR: CD8+ T cells restrict Yersinia pseudotuberculosis infection: bypass of anti-phagocytosis by targeting antigen-presenting cells. PloS Pathog 5(9): e1000573, doi:10.1371/journal.ppat.1000573, 2009.Â
4- Brown CC, Baker DC, Barker IK: Alimentary system. In: Jubb, Keneddy and Palmer's Pathology of Domestic Animals, vol.2 ed. Maxie MG, 5th ed., pp.204-205. Elsevier Limited, Philadelphia, 2007.Â
5-Fukushima H, Matsuda Y, Seki R, Tsubokura M, Takeda N, Shubin FN, Paik IK, Zheng XB: Geographical heterogeneity between Far Eastern and Western countries in prevalence of the virulence plasmid, the superantigen Yersinia pseudotuberculosis-derived mitogen, and the high-pathogenicity island among Yersinia pseudotuberculosis strains. J.Clin. Microbiol. 39(10) : 3541-3547, 2001.Â
6- Manning PJ, Wagner JE, Harkness JE: Biology and diseases of guinea pigs. In: Laboratory animal medicine, ed. Fox JG, Cohen BJ, Loew FM, pp. 162-163. Academic press, London, England, 1984.Â
7- Percy DH, Barthold SW: Pathology of laboratory rodents and rabbits, 3rd ed., pp. 225-232. Blackwell Publishing, Ames, Iowa, USA, 2007.Â
8-Vlahou G, Schmidt O, Wagner B, Uenlue H, Dersch P, Rivero F, Weissenmayer BA: Yersinia outer protein YopE affects the actin cytoskeleton in Dictyostelium discoideum through targeting multiple Rho family GTPases. BMC Microbiol. 9:138, 2009.Â