CASE IV: 19GH024 (JPC 5155510)
Signalment:
Sixteen-year-old, male, cynomolgus monkey (Macaca fascicularis).
History:
Over a 2-year period this monkey had variably increased body weight (BW) of up to 4 kilograms (kgs) greater than his original BW of 7 kgs. The following year, this monkey lost 14% of his body weight. The clinical chemistry evaluation showed significant increases in serum and urine glucose (Glu), as well as in serum triglycerides (Trigl), cholesterol (Chol) and decreases of potassium (K). There were also minimal increases in alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Although this monkey had no changes in appetite or mental faculties, he was euthanized because of clinical signs of Type 2 diabetes mellitus (T2DM). All procedures were approved by the animal care and use committee in accordance with federal regulations and the Guide for the Care and Use of Laboratory Animals.
Gross Pathology:
At necropsy, there were no gross findings.
Samples of adrenal, aorta, brain, gall bladder, heart, kidneys, liver, lung, pancreas, sciatic nerve, skin, spleen, ureters, and urinary bladder were collected and fixed in 10% neutral buffered formalin; eyes and optic nerves were fixed in Davidson's solution. These tissues were processed to slides and stained with Hematoxylin and eosin (HE) for light microscopic examination.
Laboratory results:
|
Serum |
Urine |
Body weight |
||||
|
Glucose |
Cholesterol |
Triglycerides |
Phosphorus |
Glucose |
||
|
Date/Range |
42-157 mg/dL |
20-91 mg/dL |
69-139 mg/dL |
3.7-7.8 mg/dL |
mg/dL |
~ 8 kg |
|
2017 |
59-74 |
39-154 |
66-96 |
4.6-4.9 |
NE |
8-8.4 |
|
2018 |
78-137 |
104-177 |
79-80 |
3.7-4.5 |
NE |
8.3-10 |
|
2019 |
295-501 |
933-1568 |
104-207 |
2.8-3.4 |
>1000 |
7.7 |
|
Table 1. Clinical pathology parameters and total body weights taken in this monkey from 2017 to 2019. Bolded red values are significant changes. NE= No evaluated. |
Table 1 is showing clinical chemistry parameters from 2017-2019 taken for this monkey. At the time of euthanasia, there were marked increases blood Glucose (295-501 mg/dL), urine Glucose (> 1000 mg/dL) and Chol (944-1568 mg/dL) and increases in and Trig= (105-207 mg/dL). Insulin and hemoglobin A1c were not evaluated.
Microscopic Description:
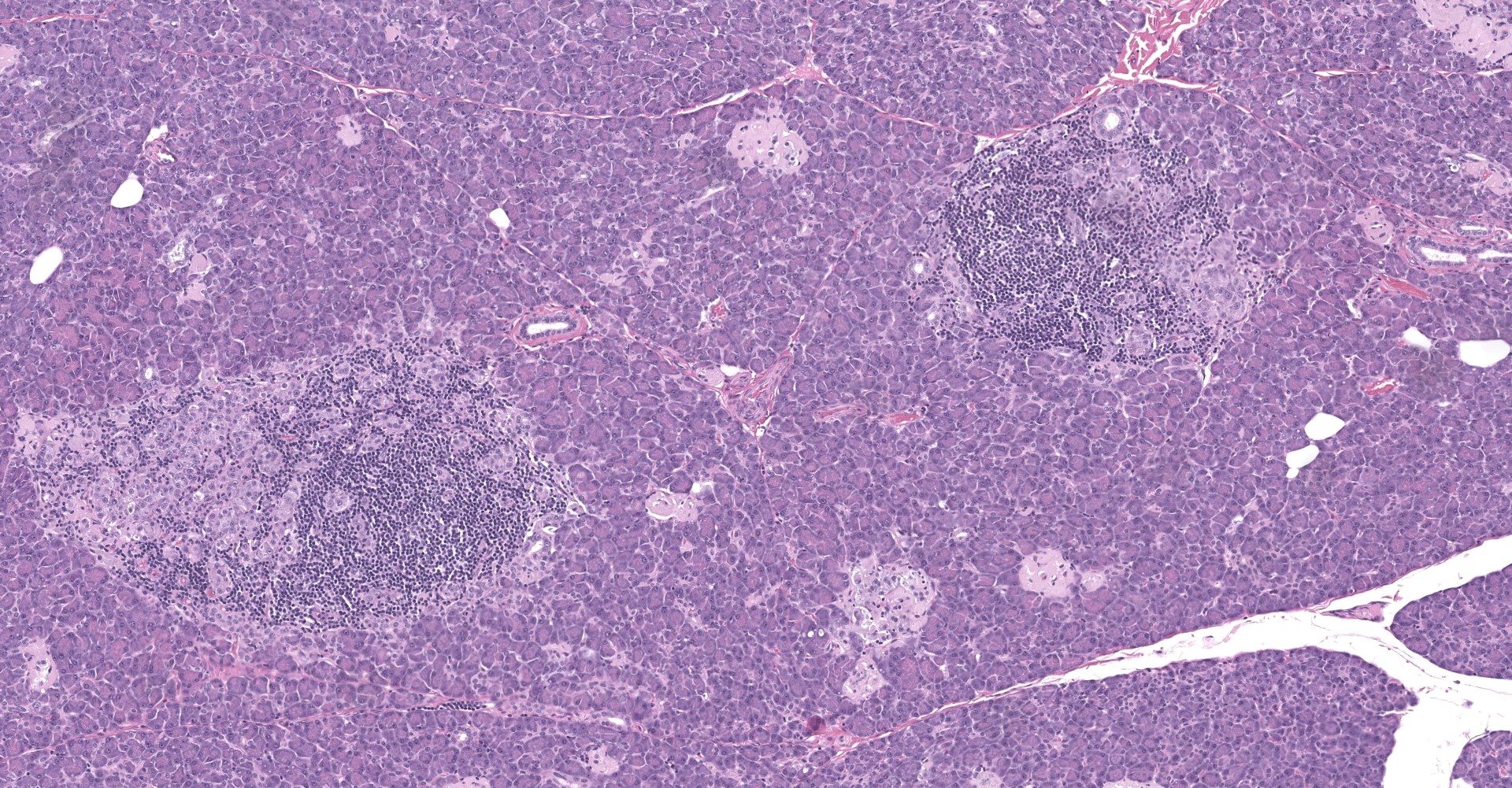
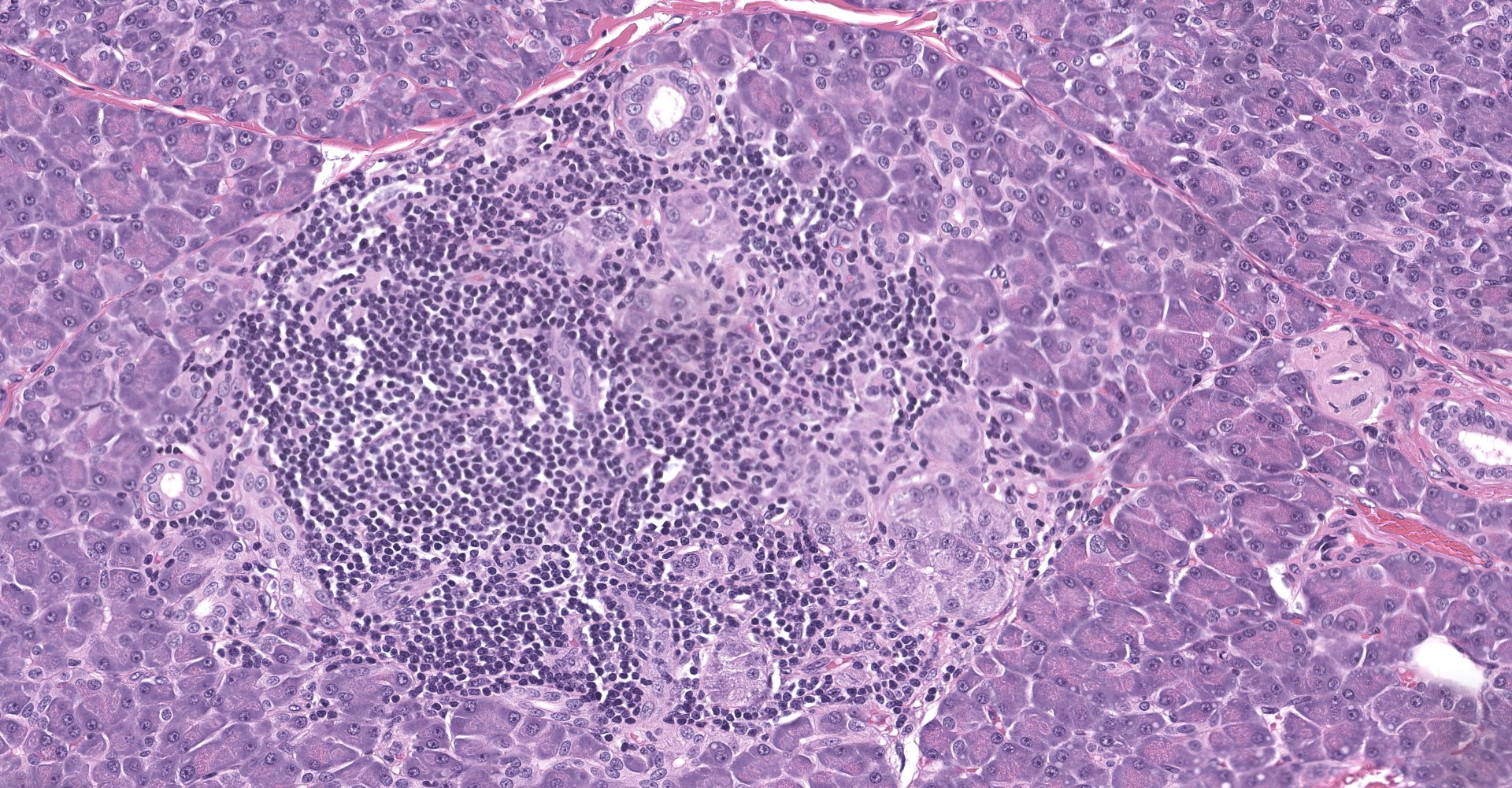
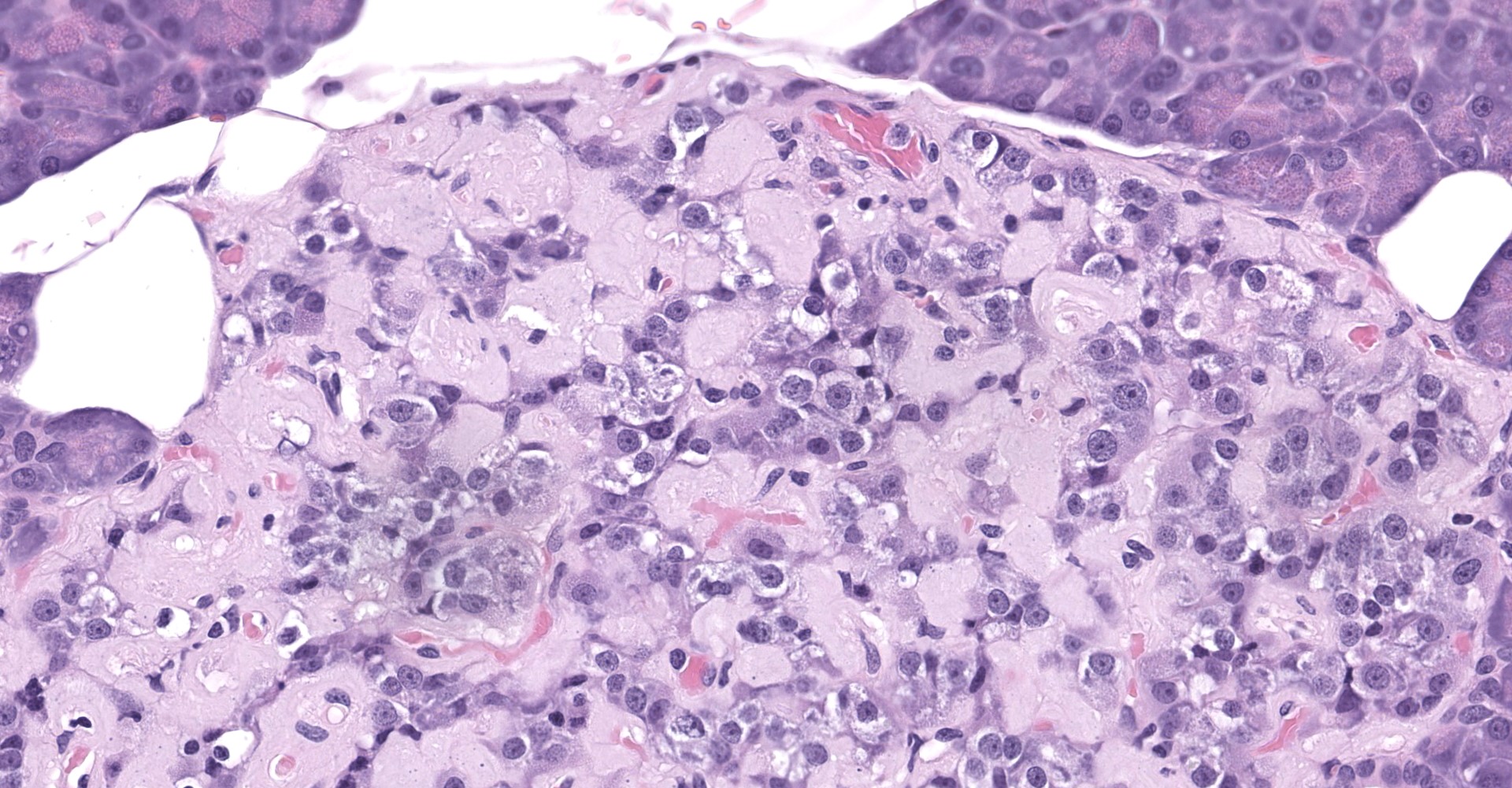
Pancreatic islets of Langerhans displayed several different histopathologic alterations. These included islet cell hyperplasia/hypertrophy, degeneration, necrosis and loss; accumulation of intracellular and extracellular pale, eosinophilic, irregularly shaped material (amyloid); and mononuclear cell infiltrate (lymphocytes). Islet cell hyperplasia/hypertrophy was characterized by increased numbers of enlarged cells with large prominent nuclei arranged in acini formations.
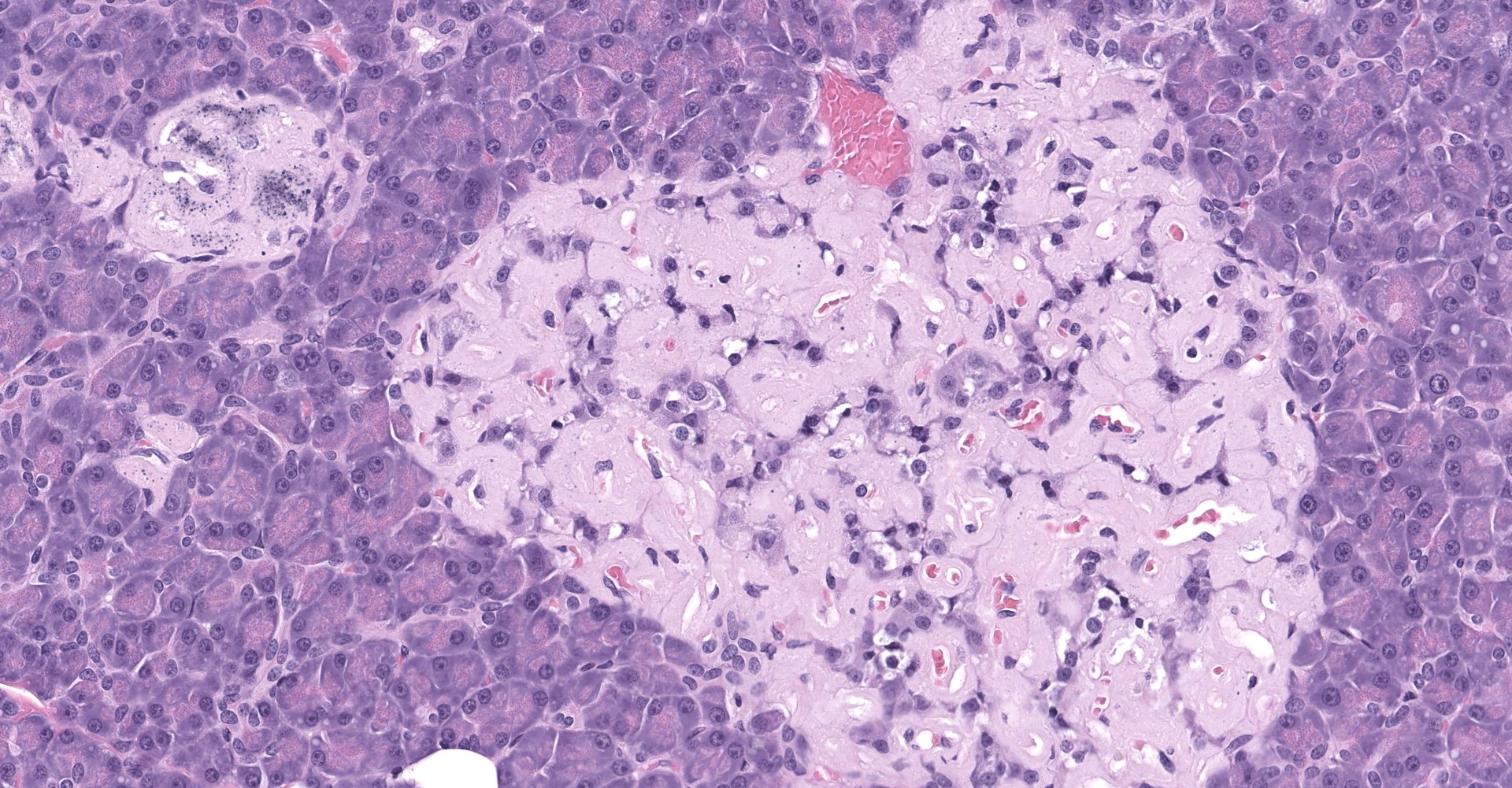
Degenerating islet cells had vacuolated cytoplasm or contained large amounts of intracytoplasmic amyloid. Necrotic cells had pyknotic to karyorrhectic nuclei. There were also several small islets that contained mostly extracellular amyloid and a few cells. A few islets of Langerhans contained variable numbers of mononuclear cells (lymphocytes). The eosinophilic material was not birefringent when the HE and Congo Red stained slides of pancreas were placed under polarized light but was still considered to be consistent with amyloid.
To further characterize the pancreatic lesions, immunohistochemical stains for Insulin (Dako Guinea Pig anti-Insulin A0565 1/7,500, 20' @ RT) and Glucagon (Abcam anti-Glucagon ab92517 1/20,000 15' @ RT) were performed on the pancreas of this monkey and from in internal control Insulin stains for beta cells demonstrated moderate to marked decreases in expression in the reported monkey as compared to the control monkey. Glucagon stains for alpha cells in the reported monkey were inconclusive because the control pancreas contained a large percentage cells that were positive for glucagon, likely due to antigen cross reactivity.
Other microscopic findings included multifocal cortical infarcts and multifocal glomerulopathy in the kidneys, and focal extensive, moderate interstitial fibrosis with cardiomyocyte loss and hypertrophy in the heart.
There were no microscopic findings in the adrenal glands, aorta, brain, gall bladder, liver, lung, sciatic nerve, skin, spleen, ureters and urinary bladder.
Contributor's Morphologic Diagnoses:
1.
Pancreas, islets of Langerhans:
Hyperplasia/hypertrophy, degeneration/necrosis, and mononuclear infiltrate
2. Pancreas, islets of Langerhans: intracellular and extracellular amyloid accumulation, marked, multifocal to diffuse
3. Pancreas, islets of Langerhans: Decreased immunohistochemical insulin expression
4. Kidneys: Cortical infarcts, chronic, multifocal and glomerulopathy (not submitted for WSC)
5. Heart: Fibrosis and cardiomyocyte loss and hypertrophy of the left myocardial wall, moderate and focally extensive (not submitted for WSC)
Contributor's Comment:
The clinical history of increases in BW followed by significant decreases of BW and the age of this monkey, as well as the alterations in the clinical pathology parameters and microscopic findings in the pancreas are all consistent with T2DM.
Factors such as obesity, sedentary lifestyle, and an increased in aging population have increased the prevalence of T2DM in humans around the world.4 Nonhuman primates (NHPs), as well as cats, dogs, and pigs also develop spontaneous type 2 diabetes mellitus (T2DM) so they are used as animal models.5 Though, they do not develop all the characteristics of T2DM in humans, they can be used to study some specific conditions.4 Depending on the stage of disease, T2DM is characterized by obesity, glucose intolerance, insulin resistant, dyslipidemia, hypertension, inflammatory processes, abnormalities of the blood coagulation system and/or pancreatic pathology.4
In NHPs, T2DM has been described in both old and new-world monkeys.6,5,15,16,1,10,13,2 The most extensive research has been conducted in cynomolgus and rhesus macaques5. In NHPs, spontaneous T2DM increases in incidence and severity with age and increased in body weight.16,1 Wagner et al., 200116 reported that approximately 40% of cynomolgus macaque 16 years of age or older had evidence of T2DM.
T2DM in humans and animal models is characterized by the following phases: Normal glucose tolerance followed by insulin resistant due to compensatory increases in insulin secretion plus alterations of carbohydrate metabolism. The disease progresses to impaired glucose with increases in fasting plasma glucose. Hyperglycemia due to decreases of insulin production caused by pancreatic pathology.4,6,16
Cynomolgus macaques are typically overweight early on in the course of the disease and as the disease gets more severe they lose weight. They also exhibit clinical chemistry alterations (increased total cholesterol, triglycerides, and free fatty acids as well as decreased HDL cholesterol), inflammation and increased blood pressure.16
The microscopic findings of T2DM in humans and NHPs include hyperplasia and hypertrophy of β cells because the pancreas responds to peripheral insulin resistance by increasing insulin production.11,9,15,16,16,17,18 As the insulin demands continue, there is co-secretion of Amylin (islet amyloid polypeptide or IAPP), resulting in intracellular accumulation within the Islets. Aggregated IAPP has cytotoxic properties causing degeneration, necrosis and loss of β cells, resulting in release of the amyloid. Infiltration of mononuclear cells is not uncommon, secondary to cellular destruction. As these lesions progress, the remaining (Alpha, delta, pancreatic polypeptide, epsilon) pancreatic cells undergo degeneration and necrosis
It is important to mention the cell types present in pancreatic Islets and what they secrete.8 Alpha cells produce Glucagon. Glucagon stimulates glycogenolysis, gluconeogenesis, lipolysis. Beta cells produce Insulin and islet-amyloid polypeptide (IAPP - amylin). Insulin stimulates glucose uptake and glycogenesis in response to hyperglycemia. Insulin is also an anabolic hormone released by beta cells in response to glucose and other nutrients such as amino acids, fatty acids and hormones. Amylin suppresses glucagon production and inhibits gastric digestive function. Delta cells produce Somatostatin. Somatostatin inhibits release of glucagon, insulin and pancreatic polypeptide. PP cells produce Pancreatic polypeptide. Their function is to antagonize cholecystokinin. Cholecystokinin inhibits exocrine pancreas secretion and gall bladder contraction and delays gastric emptying. Epsilon cells produce Ghrelin. This hormone stimulates the appetite and suppresses insulin secretion.
With immunohistochemical staining we observed remarkable decreases in insulin staining in the pancreatic islets of our 17-year old monkey, compared to a control. In 1986, Howard and Bueren7 performed computerized photometric method on immunohistochemically stained, Insulin (β cells), glucagon (α cells) and somatostatin (δ cells) cells in the pancreas of nondiabetic (ND), hormonally impaired, borderline diabetic (D) and diabetic of Macaca nigras species. He found alterations in percentages of secretory cells correlated with several of the metabolic and clinical changes. These consisted of decreases of insulin from 77% in ND to 1% in D monkeys. Glucagon and somatostatin stains also decreased in the D monkeys but in a lower degree, as percentage of these cells is normally lower in the pancreas.
Kidney and heart findings are common aged-related findings in older monkeys.
Contributing Institution:
Web site address):
Pfizer Drug Research and Development
555 Eastern Point Rd
Groton, CT 06450
JPC Diagnosis:
1. Pancreas, islets: Hyperplasia, diffuse, moderate with islet cell hypertrophy and rare tubuloinsular complexes (nesidioblastosis).
2. Pancreas, islets: Amyloidosis, diffuse, severe.
3. Pancreas, islets: Insulitis, lymphocytic, multifocal, moderate.
JPC Comment:
The contributor expertly summarizes the pathogenesis, clinical findings, and nuances associated with T2DM in NHPs, which closely mirror those of humans.
The prevalence of T2DM has significantly increased amongst people throughout the world and the trend will likely continue for the foreseeable future.3 In addition to characteristic features provided by the contributor (e.g. obesity, hypertension), human patients with T2DM are predisposed to two neurodegenerative diseases in which misfolded amyloidogenic peptides play pivotal roles, Alzheimer's disease and Parkinson's disease. Ongoing research has found insulin-degrading enzyme (IDE) to be a link between T2DM and these two diseases.12
IDE has degradative activity over insulin, playing a central role in its clearance and regulation, and therefore overall homeostasis. First demonstrated in mice with an ablated Ide gene, its absence or loss of function results in hyperinsulinemia and contributes to the development of T2DM.12
In addition to degrading insulin, IDE interacts with and degrades a wide spectrum of substrates, including islet amyloid polypeptide (IAPP, also known as amylin) and amyloid-β (Aβ) peptides, which have the ability to undergo conformational change and form β-sheets. Furthermore, the enzyme is concentrated in tissues where amyloidogenic risk is elevated, such as pancreatic β cells and the brain, which are continuously challenged with IAPP and Aβ aggregation, respectively. One way IDE prevents formation of these aggregates is by exhibiting chaperone-like activity over amyloidogenic peptides and facilitating proper folding, including amyloidogenic peptides related to neurodegenerative diseases, such as Aβ in the case of Alzheimer's disease and α-synuclein, which plays a pivotal role in the development of Parkinson's disease.12
T2DM's association with the development of Alzheimer's disease and Parkinson's disease is potentially tied to hyperinsulinemia, which occurs in the early stages in the disease process as described by the contributor. High levels of insulin may overwhelm IDE, resulting in favorable conditions for the aggregation of amyloidogenic peptides.12
Given its association with these three entities, IDE is an enticing target for medicinal research, although challenges, such as those due to toxicity (e.g. hypoglycemia) must be overcome.12
During the conference, participants noted what the contributor described within rare islets as hyperplastic and hypertrophic cells arranged in acini formations. The moderator discussed how this finding is consistent with nesidioblastosis given that neither acini nor tubular structures are normally present in these locations.
Nesidoblastosis is a non-neoplastic proliferation of islet and ductular tissue within the pancreas that is histologically characterized by irregularly sized islets, hypertrophied β cells, and ductulo-insular complexes ("insulo" referring to islet cells including but not limited to, insulin producing β cells).5 The pathogenesis of nesidioblastosis is unclear; it occurs as both a regenerative response to pancreatic insults and in response to increased β cell trophic factors, such as T2DM.
Nesidioblastosis is the most common cause of hyperinsulinemic hypoglycemia in humans.5 However, nesidioblastosis is sporadically reported in domestic species, particularly in dogs, and is typically an incidental finding.8
References:
1. Cai G, Cole SA, Tejero ME, Proffitt JM, Freeland-Graves JH, Blangero J, Comuzzie AG. Pleiotropic effects of genes for insulin resistance on adiposity in baboons. Obes Res. 2005;12(11):1866?72.
2. Cann JA, Kavanagh K, Jorgensen MJ, Mohanan S, Howard TD, Gray SB, Hawkins GA, Fairbanks LA, Wagner JD. Clinico-pathologic characterization of naturally occurring diabetes mellitus in vervet monkeys. Vet Pathol. 2010;57(5):714?8.
3. Cefalu WT, Animal Models of Type 2 Diabetes: Clinical Presentation and Pathophysiological Relevance to the Human Condition, ILAR Journal, Volume 57, Issue 4, 2006, Pages 186?198
4. Comuzzie AG, Cole SA, Martin L, Carey KD, Mahaney MC, Blangero J, VandeBerg JL. The baboon as a nonhuman primate model for the study of the genetics of obesity. Obes Res. 2004;11(1):75?80.
5. Gacar A, Pekmezci D, Karayigit MO, Kabak YB, Gulbahar MY. Nesidioblastosis in a simmental calf. J Comp Pathol. 2012;147(4):491-494.
6. Harwood HJ Jr, Listrani P, Wagner JD. Nonhuman primates and other animal models in diabetes research. J Diabetes Sci Technol. 2012;6(4):504?515.
7. Howard CF. Diabetes in Macaca nigra: metabolic and histologic changes. Diabetologia. 1975 Nov 1;10(1):671-7.
8. Howard CF Jr, Van Bueren A. Changes in islet cell composition during development of diabetes in Macaca nigra. Diabetes. 1986;45(2):165‐181.
9. Jubb KFV, Stent AW. Pancreas. In: Jubb, Kennedy, and Palmer's Pathology of Domestic Animals Maxie, M Grant. Sixth edition. St. Louis, Missouri: Elsevier, 2016. 2:370-3.
10. Palotay JL, Howard CF: Insular Amyloidosis in Spontaneously Diabetic Nonhuman Primates, Vet Pathol. 1982;19 (Suppl 7):181-192.
11. Pirarat N, Kesdangsakolwut S, Chotiapisitkul S, Assarasakorn S. Spontaneous diabetes mellitus in captive Mandrillus sphinx monkeys: a case report. J Med Primatol. 2008;47(4):162?5.
12. Sheldon, W. G., & Gleiser, C. A. (1971). Amyloidosis of the Islets of Langerhans in a Crab-Eating Monkey (Macaca fascicularis). Veterinary Pathology, 8(1), 16?18.
13. Sousa L, Guarda M, Meneses MJ, Macedo MP, Vicente Miranda H. Insulin-degrading enzyme: an ally against metabolic and neurodegenerative diseases [published online ahead of print, 2021 Aug 16]. J Pathol. 2021;10.1002/path.5777.
14. Tardif SD, Power ML, Ross CN, Rutherford JN, Layne-Colon DG, Paulik MA. Characterization of obese phenotypes in a small nonhuman primate, the common marmoset (callithrix jacchus). Obesity (Silver Spring). 2009;18(8):1599?505.
15. Wagner JD, Cann JA, Zhang L, Harwood HJ Jr. Diabetes and obesity research using nonhuman primates. In: Abee CR, Mansfield K, Tardif SD, Morris T, eds. Nonhuman primates in biomedical research Volume 2: Diseases. 2nd ed. 2012. Elsevier. Chapter 15, pp 699?742
16. Wagner JD, Cline JM, Shadoan MK, Bullock BC, Rankin SE, Cefalu WT. Naturally occurring and experimental diabetes in cynomolgus monkeys: a comparison of carbohydrate and lipid metabolism and islet pathology. Toxicologic pathology. 2001 Jan;29(1):152-8.
17. Wagner JD, Kavanagh K, Ward GM, Auerbach BJ, Harwood HJ Jr, Kaplan JR, Old World Nonhuman Primate Models of Type 2 Diabetes Mellitus, ILAR Journal, Volume 57, Issue 4, 2006, Pages 259?271.
18. Westermark P. Amyloid in the islets of Langerhans: thoughts and some historical aspects. Upsala journal of medical sciences. 2011 May 1;116(2):819
19. Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiological reviews. 2011 Jul;91(4):795-826.