Signalment:
Two-year-old, hermaphrodite, veiled chameleon, (
Chamaeleo calyptratus).Initially the
chameleon exhibited open mouth breathing and declining appetite, eventually
requiring forced feeding. The patient returned to the clinic 4 weeks later with
severe dehydration and obvious weight loss. The chameleon was euthanized at
that time.
Gross Description:
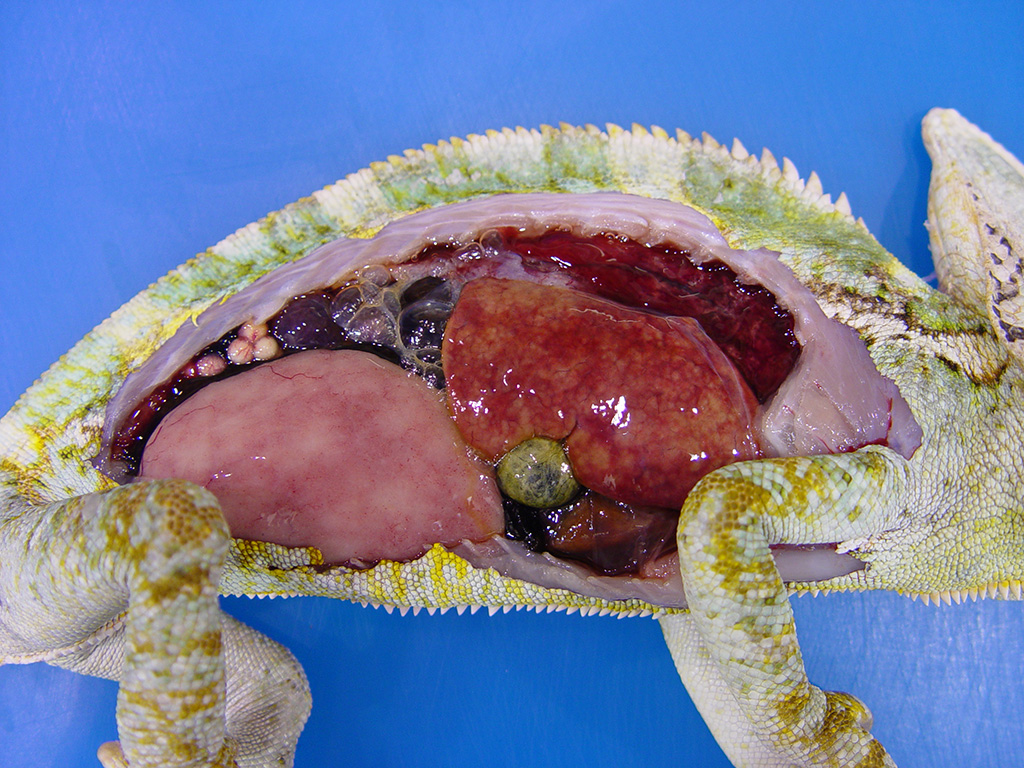
The
chameleon was in good nutritional condition with normal muscle mass and
moderate coelomic adipose stores. A small amount of clear red-tinged fluid was
present in the coelom. Diffuse red discoloration was evident in the expanded
lungs and the liver was mottled tan and red with slight rounding of the
margins. Ovotestes were present bilaterally. Mucoid content was evident in the
lumen of the stomach and the colon; the intestinal content was otherwise scant.
Histopathologic Description:
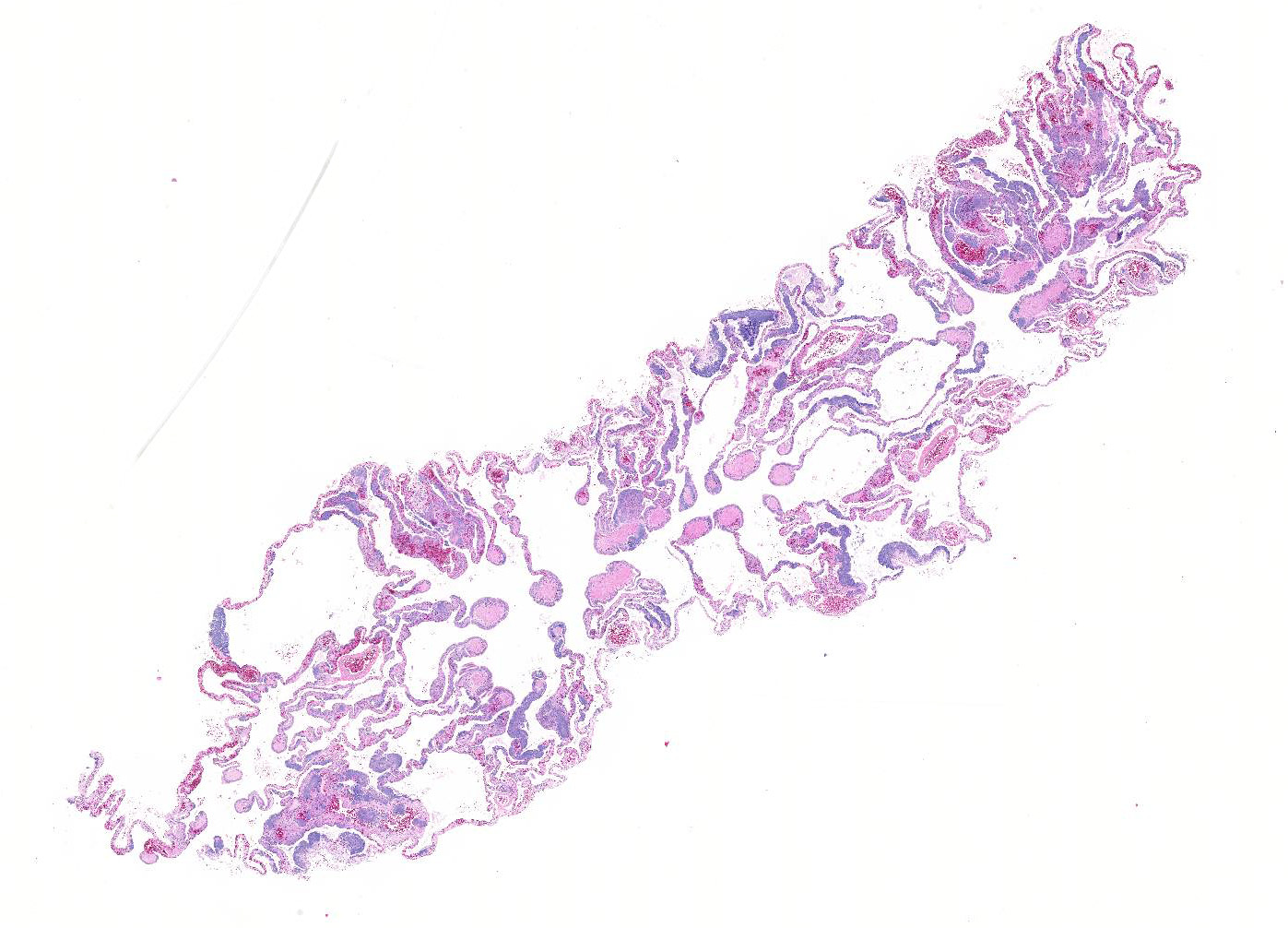
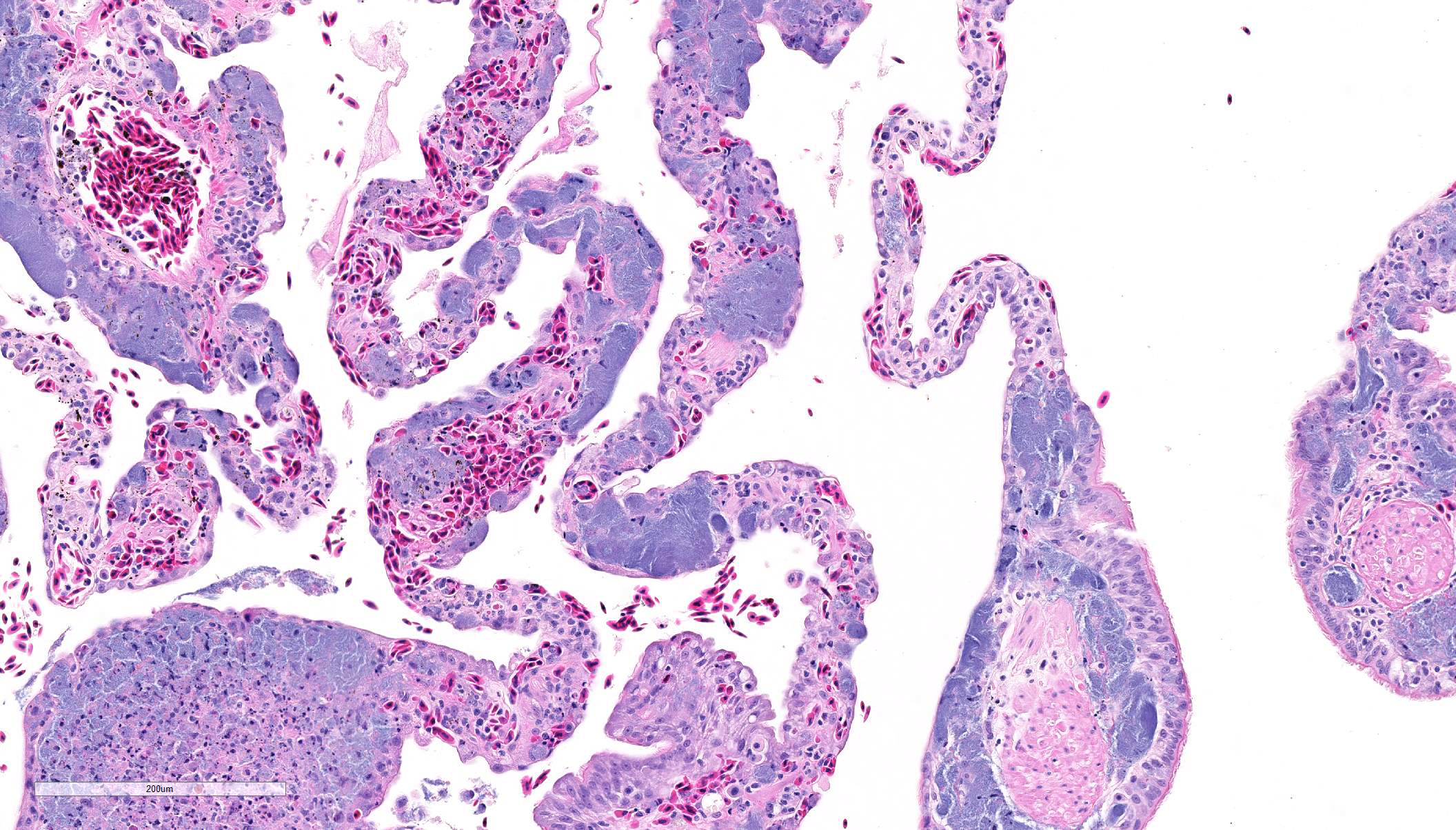
Lung:
The faveolar septa are expanded with moderate to marked congestion of the
vasculature and myriad slender bacilli are present within the lumina of blood
vessels; bacteria are both free within the lumina and present within the
cytoplasm of macrophages. Hemorrhage, intravascular fibrin thrombi and necrotic
cellular debris often accompany the bacterial colonies and scattered small
aggregates of epithelioid macro-phages are present multifocally in the septa.
In areas, there is necrosis of the faveolar epithelium and erythrocytes,
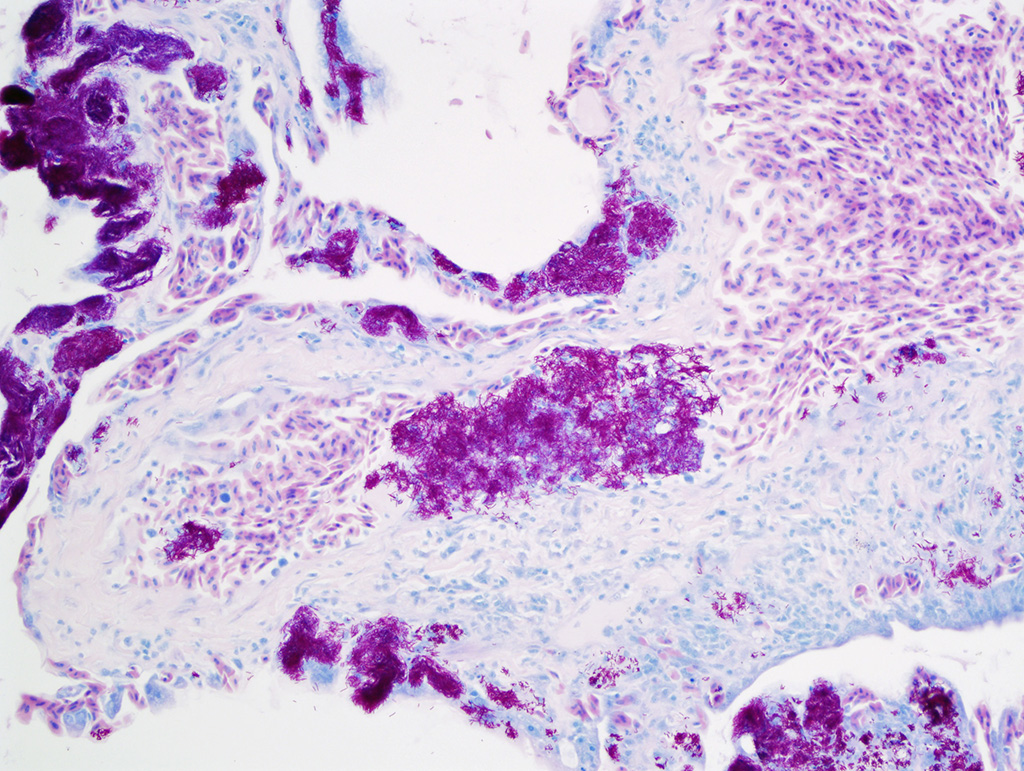
proteinaceous fluid and cellular debris are noted with the faveolar spaces. The bacteria are acid-fast
with the Ziehl-Neelsen stain. Similar
intravascular bacteria and occasional small aggregates of macrophages
containing bacteria are present in most organs (liver, pancreas, kidney, brain,
spleen, skeletal muscle, adrenal glands, small intestine and ovotestes).
Morphologic Diagnosis:
Necrotizing and histiocytic
interstitial pneumonia, diffuse, subacute, with myriad
intravascular/intrahistiocytic acid-fast bacilli and intravascular fibrin
thrombi
Lab Results:
Sections of lung were
submitted for bacterial culture. Primary culture on a sheep
blood agar plate after 48 hour aerobic incubation at 37
oC
resulted in heavy growth of tiny smooth cream-colored colonies. Grams
stain revealed pleomorphic gram-positive bacilli. The
bacteria were acid-fast with the Ziehl-Neelsen stain. The
isolate was sent to the National Reference Centre for Mycobacteriology (Public
Health Agency of Canada). Based on
16s and
hsp65 gene
sequencing, the isolate had 100% sequence identity to
Mycobacterium
chelonae chemovar
niacinogenes.
Condition:
Pneumonia/Mycobacterium chelonae
Contributor Comment:
Mycobacteria
are ubiquitous in nature and can be isolated from the soil, dust, water and
bioaerosols.
1 Reptiles are generally thought to acquire
mycobacterial infections via ingestion or through defects / penetrating injury
in the skin.
11 In this chameleon, there was a localized area of
intestinal ulceration and granulomatous enteritis, suggesting that infection
may have been acquired through the intestinal tract. In
reptiles, like most species, mycobacterial infections tend to be chronic with
rare acute infections reported.
5 The typical gross lesions are
grey-white nodules in multiple organs. Microscopically, early lesions are
composed of organized collections of foamy macrophages that with time may
become chronic granulomas composed of a mixture of epithelioid macrophages,
lymphocytes, plasma cells, and multinucleated giant cells often surrounding a
central region of necrosis and occasionally with a surrounding wall of fibrous
connective tissue.
11 In this case, there were a few small early
granulomas in multiple organs including the brain, lung, liver, kidneys and
intestine. More striking, however, was the presence of myriad intravascular
bacteria in multiple organs and frequent intravascular fibrin thrombi. These
changes are compatible with acute to subacute infection with acute bacteremia
and disseminated intravascular coagulation.
Mycobacteria are
broadly divided into two groups:
Mycobacterium tuberculosis complex and
non-tuberculous myco-bacteria.
2 While only non-tuberculous
mycobacteria have been reported to cause infections in reptiles, several
different species have been associated with these infections. These include
M.
confluentis,
M. chelonae, M. haemophilum, M. hiberniae, M. neoarum, M.
confluentis, M. nonchromogenicum, M. marinum, and M. thamnopheos.
2,5,11
The most common causes of mycobacteriosis in reptiles are reported to be
M.
marinum,
M. chelonae and
M. thamnopheos.
5 The
isolate in this case was confirmed to be
M. chelonae chemovar
niacinogenes.
Non-tuberculous
mycobacteria are classified into four Runyon groups according to growth rate
and pigmentation.
5 Runyon group I mycobacteria are slow growing and
form pigment in the light following growth in the dark. Runyon group II
organisms are also slow growing bacteria; these bacteria form pigment in the
dark following growth in the light. Runyon group III bacteria are slow growing
and do not form pigment in the dark or light. Fast growing non-pigmented
mycobacteria are placed in Runyon group IV. These mycobacteria form mature
colonies on solid agar within 7 days, while bacteria in Runyon groups I to III
take longer periods of time for cultivation.
2 Most mycobacteria which
infect reptiles fall into Runyon groups I and IV.
5 Mycobacterium
chelonae is a rapidly growing mycobacteria belonging to Runyon group IV.
4
Rapidly growing mycobacteria are relatively resistant to standard disinfectants
and antibiotic treatment and are increasingly recognized as opportunistic
pathogens in humans.
1,2
In
reptiles
, M. chelonei has been reported in association with
osteoarthritis
in a Kemps Ridley sea turtle,
4 with stomatitis and subcutaneous
granulomas in a boa constrictor,
6 and with disseminated infection in
a loggerhead sea turtle
8 and a veiled chameleon.
9 There
is a single report of this bacterium causing acute fatal sepsis and
disseminated intravascular coagulation in an eastern spiny softshell turtle
8
with lesions very similar to those described in this case. Because of poor
response to treatment and the zoonotic potential, euthanasia is often
recommended for reptiles with myco-bacterial infections.
JPC Diagnosis:
Lung: Pneumonia, histiocytic and necrotizing, multifocal to
coalescing, moderate, with numerous intrahistiocytic and intravascular bacilli, veiled
chameleon,
Chamaeleo calyptratus.
Conference Comment:
The contributor provides an outstanding review of non-tuberculous
Mycobacteria in reptiles.
Mycobacteria spp. are a large genus
comprised of over 100 species of obligate pathogenic, potentially pathogenic,
and environmental saprophytic bacteria.
6 They are all
morphologically similar and are composed of aerobic, gram-positive, acid-fast,
non-spore forming bacilli.
6 Conference participants were impressed
by the large numbers of intrahistiocytic and intravascular thin filamentous
bacilli that stain slightly basophilic on hematoxylin and eosin stained tissue
section. These bacilli are intensely acid-fast positive with Fite-Faraco and
Ziehl-Neelsen acid-fast stains, run by the Joint Pathology Center prior to the
conference.
Mycobacterium chelonae infection,
confirmed by the contributor as the cause of rapid disseminated disease in this
animal, is an opportunistic and potentially zoonotic pathogen that is
characterized by rapid growth and high resistance to antibiotics.
1,2
As mentioned by the contributor, spontaneous non-tuberculous
Mycobacteria
sp., including
M. avium,
M. chelonae,
M. szulagai,
M.
fortuitum,
M. marinum,
M. hemophilum,
M. kansasii, and
M. ulcerans have been reported to be the classic etiologic agents that
cause histiocytic granulomas snakes, turtles, lizards, and crocodiles and
should top the list of differential diagnoses for lesions similar to this case.
2,5,10,11
The obligate intracellular bacteria,
Chlamydophila pneumoniae, can also
occasionally infect reptilian species and induce histiocytic granulomas and
should be considered as a differential diagnosis. Additionally, relatively
recently described Chlamydia-like bacteria
Parachlamydia acanthamoebae
and
Simikania negevensis, have been sporadically reported to form
histiocytic granulomas in reptiles as well.
11
While
histiocytic granulomas in reptiles are often induced by intracellular bacteria,
such as in this case, heterophilic granulomas in reptiles are caused by
extracellular pathogens, including most bacterial and fungal etiologies. Tissue
injury can also induce heterophilic granulomas in reptiles.
5
Heterophilic granulomas are characterized by accumulation and degranulation of
heterophils leading to a central area of necrosis, stimulating a strong
macrophage foreign body-like response. Caseocalcareous nodules, lymphoid
infiltration, and peripheral fibrosis, typical of mammalian granulomas, have
not been observed in reptilian heterophilic granulomas.
5 Both
histiocytic granulomas and heterophilic granulomas can progress to chronic
granulomas, characterized by a fibrous connective tissue capsule, lymphocytes
and plasma cell infiltration, and a central area of necrosis with a prominent
lamellated appearance.
5
References:
1. De Groote MA, Huitt G. Infections
due to rapidly growing
Mycobacteria.
Clin Infect Dis. 2006;
42:175663.
2. Ebani,
VV, Fratini F, Bertelloni F, et al. Isolation and identification of
Mycobacteria
from captive reptiles.
Res Vet Sci. 2012; 93:11361138.
3. Fremont-Rahl
JJ, Ek C, et al.
Mycobacterium liflandii outbreak in a research colony
of Xenopus (Silurana) tropicalis frogs.
Vet Pathol. 2011; 48(8):856-867.
4. Greer
LL, Strandberg JD, Whitaker BR.
Mycobacterium chelonae osteoarthritis in
a Kemp's Ridley Sea Turtle (
Lepidochelys kempii).
J Wildl Dis. 2003;
39(3):736-741.
5. Jacobson,
ER. Bacterial diseases of reptiles. In: Jacobson ER, ed.
Infectious Diseases
and Pathology of Reptiles: Color Atlas and Text. Boca Raton, Florida: CRC
Press; 2007: 461526.
6. Mauldin E,
Peters-Kennedy J. Integumentary system. In: Maxie MG, ed.
Jubb, Kennedy, and
Palmers Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia,
PA:Elsevier; 2016:639-641.
7. Mitchell MA. Mycobacterial infections in reptiles.
Vet
Clin Exot Anim. 2012; 15(1):10111.
8. Murray
M, Waliszewski NT, Garner MM, et al. Sepsis and disseminated intravascular
coagulation in an eastern spiny softshell turtle (
Apalone spinifera
spinifera) with acute mycobacteriosis.
J Zoo Wildl Med. 2009;
40(3):572-575.
9. Nardini
G, Florio D, DiGirolamo N, et al. Disseminated mycobacteriosis in a stranded
Loggerhead Sea Turtle (
Caretta caretta).
J Zoo Wildl Med. 2014;
45(2): 357360.
10. Reavill
DR, Schmidt RE. Mycobacterial lesions in fish, amphibians, reptiles, rodents,
lagomorphs, and ferrets with reference to animal models.
Vet Clin Exot Anim.
2012; 15(1): 2540.
11. Soldati
G, Lu ZH, Vaughan L, et al. Detection of
Mycobacteria and
Chlamydiae
in granulomatous inflammation of reptiles: A retrospective study.
Vet Pathol.
2004; 41(4):388397.