CONFERENCE 10, CASE I:
Signalment:
Mature ewe, Ovis aries, ovine.
History:
The cadaver and viscera of a mature ewe underwent routine meat inspection by an abattoir veterinarian who noted an emaciated cadaver, abnormal kidneys and liver. The liver was donated to the Department of Veterinary Medicine, University of Cambridge, for veterinary public health teaching and was passed to the anatomic pathology service for further investigation.
Gross Pathology:
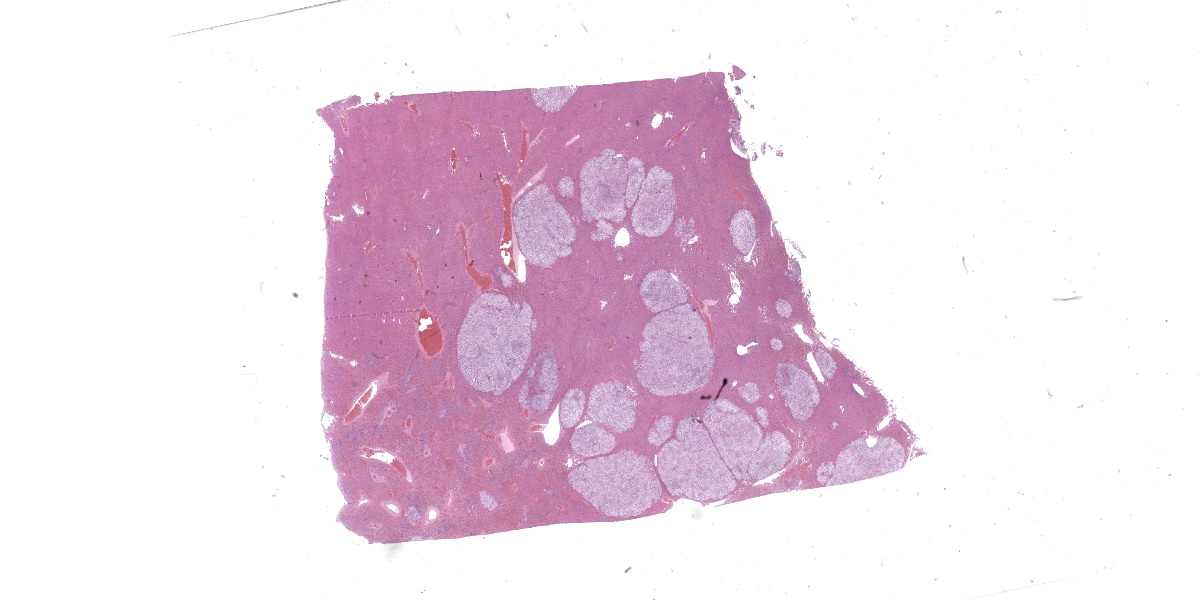
The liver is expanded by multifocal to coalescing, well-demarcated, cream nodules that are unencapsulated and infiltrative. The nodules are up to 25 mm diameter.
Microscopic Description:
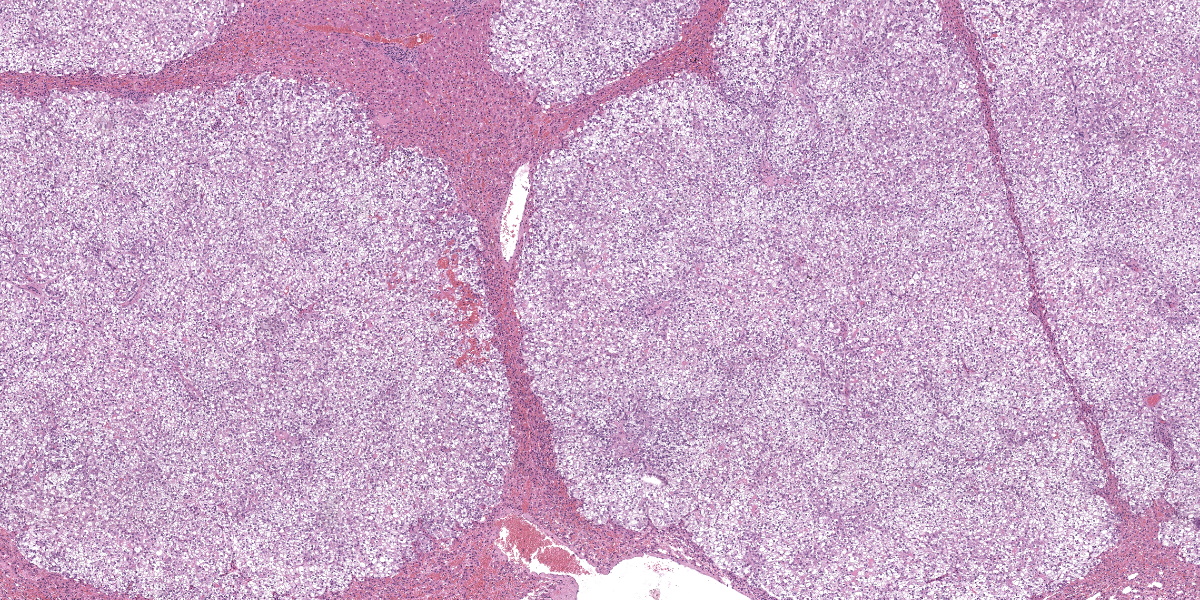
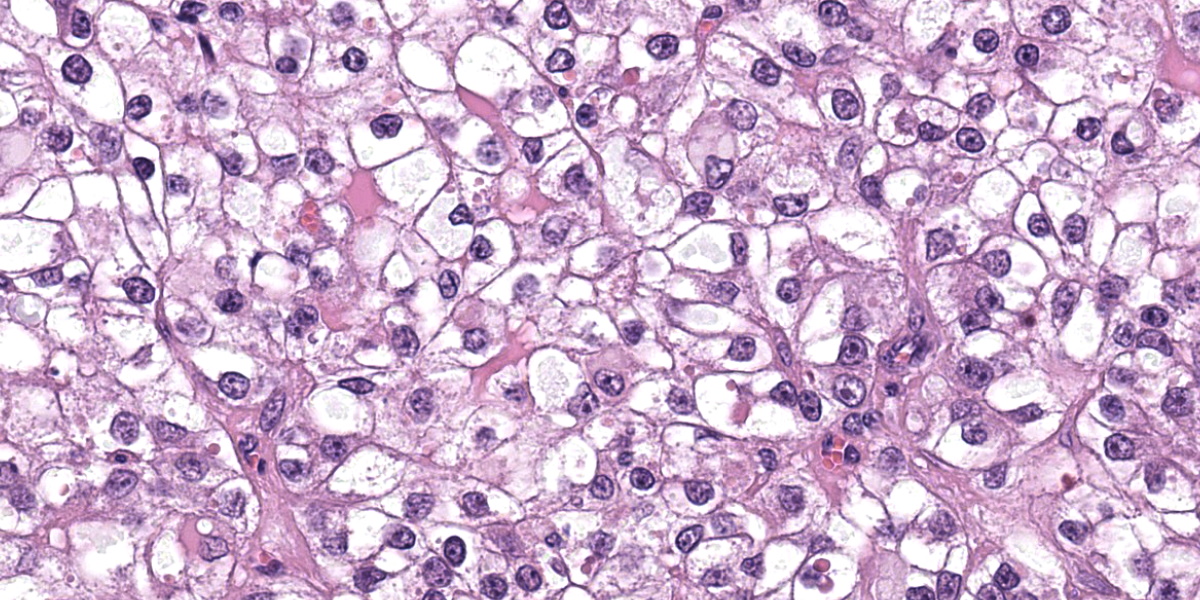
Liver: Apparent sub-grossly within the hepatic parenchyma forming multifocal to coalescing nodular masses is an unencapsulated moderately densely cellular neoplasm that is infiltrative, with small neoplastic nodules infiltrating the surrounding hepatic parenchyma. The neoplastic cells are arranged in sheets supported by a moderately fine collagenous stroma with blood vessels. The neoplastic cells are polygonal and moderately sized, with clearly demarcated cellular boundaries and a moderate amount of cytoplasm that is clear, or exhibits either globular eosinophilic deposits, or fine fibrillar eosinophilic strands, that are PAS-positive. The nuclei are round and generally centrally placed within the cells, with lightly stippled chromatin and frequently a single prominent basophilic nucleolus. There is one mitosis per ten high power fields (per 2.37 mm2). There is a moderate degree of anisocytosis and anisokaryosis. There are small foci of necrosis and haemorrhage, comprising less than 5% of the total area of neoplasm in the section examined, and scattered rare neoplastic cells exhibit apoptosis, characterised by shrunken brightly eosinophilic cytoplasm and nuclear pyknosis.
The hepatic parenchyma immediately adjacent to the neoplastic nodules is multifocally compressed. Small numbers of lymphocytes and rarer plasma cells are present multifocally within portal areas, and portal areas also exhibit mild fibrosis multifocally. Deposits of clumped golden-brown granular pigment (haemosiderin) are present multifocally in hepatic Kupffer cells.
Bile duct epithelium exhibits strong positive cytoplasmic immunohistochemical reactivity to cytokeratin 19 (positive internal control tissue). The neoplastic cells do not exhibit positive staining with cytokeratin 19.
Contributor?s Morphologic Diagnosis:
Liver: Hepatocellular carcinoma, clear cell variant.
Contributor?s Comment:
In this case, a diagnosis of the clear cell variant of hepatocellular carcinoma was made on the basis of the histologic cellular arrangement and morphology. The diagnosis was supported by the macroscopic appearance of the lesion as unencapsulated and infiltrative nodular masses.
Hepatocellular carcinomas have a widely variable histologic appearance due to different cellular arrangements, likely reflecting the degree of differentiation of the neoplastic hepatocytes.6 Four major histologic variants are described, namely trabecular, pseudoglandular, scirrhous, and solid. As the name suggests, in trabecular hepatocellular carcinomas, the predominant cellular arrangement has a resemblance to normal hepatic trabeculae, although the plates of cells may vary considerably in width, with trabeculae frequently composed of 5-10 cells thickness of neoplastic hepatocytes. In other foci the trabeculae may be quite thin, and this level of variability is a feature that may be helpful in the distinction between well-differentiated trabecular hepatocellular carcinoma and hepatocellular adenoma. In the latter, the trabeculae would be anticipated to be of uniform thickness.6 In neoplasms exhibiting the pseudoglandular pattern, the neoplastic hepatocytes form rudimentary acini, whereas in the scirrhous form there is dense connective tissue, and aggregates of neoplastic hepatocytes are infiltrated by ductular epithelium.6 As the name suggests, solid hepatocellular carcinomas are formed by solid sheets of neoplastic hepatocytes. In some variants of this sub-type, the neoplastic hepatocytes exhibit prominent cytoplasmic vacuolation and are described as clear cell hepatocellular carcinomas, as in this case.6
Clear cell hepatocellular carcinomas, or hepatocellular carcinomas that exhibit a partial clear cell pattern, have been described in a number of species including dogs,14 captive fennec foxes,13 and sheep.1 It has previously been suggested that the clear cell pattern may be associated with cytoplasmic PAS-positivity and glycogen deposition1 and PAS-positive cytoplasmic deposits are observed in the case described here.
In cases of hepatocellular carcinoma requiring immunohistochemical diagnostic confirmation, Hep Par-1 can be used to positively identify normal and neoplastic hepatocytes.6,16 In human medicine, arginase-1 has been suggested to be a more sensitive and specific marker of hepatocellular differentiation than Hep Par-1 and immunohistochemical staining with arginase-1 may therefore also be of diagnostic utility in veterinary pathology.4 Normal and neoplastic biliary epithelial cells express cytokeratin 7 and 19, and epithelial membrane antigen. These immunohistochemical staining characteristics can be utilised to distinguish hepatocellular carcinoma from cholangiocellular neoplasms in many cases although some poorly differentiated canine hepatocellular carcinomas may downregulate Hep Par-1 expression and exhibit some cytokeratin 19 immunoreactivity.6 In our case, cytokeratin 19 was utilised for additional educational confirmation that the neoplastic cells were not of biliary origin.
Fallen stock collection centres, abattoirs and meat packing plants all provide the opportunity to study the prevalence of neoplasms arising in sheep.1,2,12 Hepatocellular carcinomas appear to be more frequent than cholangiocellular neoplasms and occur in lambs as well as adult animals.1,2 Extramedullary haematopoiesis has been described as a feature of hepatic tumors in lambs aged less than six months.1,2 Although considered to be rare in domestic species, hepatoblastomas, believed to arise from hepatic stem/progenitor cells, have also been described in lambs.5,6 As in other species, the ovine liver is also a site for tumor metastases, lymphoma, and mast cell neoplasms.8,10,11
Metastasis has previously been reported in cases of ovine hepatocellular carcinoma.1 Due to the nature of the abattoir case material described here, a full post mortem examination was not conducted and the presence or absence of metastatic spread to the draining lymph nodes or other viscera could not be determined. The renal lesions were not examined microscopically but the kidneys did not display macroscopic evidence of neoplastic infiltration.
Contributing Institution:
Department of Veterinary Medicine, The Queen's Veterinary School Hospital, University of Cambridge.
Cambridge CB3 0ES, UK.
https://www.vet.cam.ac.uk
JPC Diagnosis:
Liver: Hepatocellular carcinoma, clear cell variant
JPC Comment:
This week?s moderator was Dr. Rebecca Smedley of Michigan State University who led participants through a neoplasia-centric conference. This first case was recently published in the Journal of Comparative Pathology9 and we were glad to explore it further.
From subgross, the contrast between neoplastic hepatocytes laden with glycogen (confirmed via PAS) and normal hepatocytes is stark. Several conference participants also noted hyaline inclusions within the cytoplasm of clear cell hepatocytes that were reminiscent of Mallory bodies.3,7 Mallory bodies (syn. Mallory-Denk Bodies) have been previously noted within hepatocytes of humans (and in some lab animal models) and are composed of a mix of misfolded proteins such as keratins 8 and 18, ubiquitin, and heat shock proteins.3,7 Associated conditions include alcohol-related steatohepatitis (fatty liver disease), copper hepatopathy, and hepatic neoplasms. In human cases of hepatocellular carcinoma, Mallory-Denk bodies are noted in 20-30% of cases, though these should be distinguished from hyaline bodies which are histologically similar, but lack keratin.7 IHC for P62 and other protein constituents (keratin) can aid in determination. Both elements represent hepatocellular dysfunction and aberrant protein folding.
Given the age (and of course, species) of this animal, we performed a copper (rhodanine) stain, which was negative. A reticulin stain demonstrates a normal level of staining of reticulin fibers between hepatic cords in the unaffected hepatic parenchyma in this case and very little reticulin staining within the neoplastic foci. In areas of hepatic lobular collapse or atrophy, the reticulin fibers will be closer together due to a loss of or decreased size of hepatocytes, resulting in an increase in the density of reticulin fibers. In proliferative hepatocellular foci (hyperplastic, regenerative, or neoplastic) there will be less reticulin fibers, so this stain helps to identify proliferative foci.
Copper-associated hepatopathy may be an inciting cause of HCC in some dogs, although additional research is needed to support this possibility. Additionally, we ran a HepPar-1(a hepatocyte marker) and a CK19 (a marker of biliary epithelium) to characterize the neoplastic cells, as there are reports of decreased HepPar-1 expression and increased CK19 expression in poorly differentiated tumors. These markers did not demonstrate abnormalities along these lines in the neoplasm in this case.
Finally, Dr. Smedley discussed the potential utility of glypican-3 with the group. As Glypican-3 labels neoplastic hepatocytes but not normal or hyperplastic foci,15 it would be an invaluable tool in sorting through edge cases where suspect neoplasms also contain rare portal tracts which are typically a distinguishing feature of hepatic hyperplasia. In conjunction with P-glycoprotein or arginase-1 where expression is decreased in neoplastic cells compared with normal hepatocytes, glypican-3 may prove a useful diagnostic tool.
References:
- Anderson LJ, Sandison AT. Tumors of the liver in cattle, sheep and pigs. Cancer 1968;21:289-301.
- Bundza A, Greig AS, Dukes TW. Primary Hepatocellular Tumors in Animals Killed at Meat Packing Plants: Report of 11 cases. Can Vet J 1984;25:82-85.
- Chelliah, Adeline R, Radhi, Jasim M., Hepatocellular Carcinoma with Prominent Intracytoplasmic Inclusions: A Report of Two Cases, Case Reports in Hepatology, 2016, 2032714.
- Choi WT, Kakar S. Immunohistochemistry in the Diagnosis of Hepatocellular Carcinoma. Gastroenterol Clin North Am 2017;46:311-325.
- Cotchin E. Spontaneous tumours in young animals. Proc R Soc Med 1975;68:653-655.
- Cullen JM. Tumors of the liver and gallbladder. In: Meuten DM, ed. Tumors in domestic animals. 5th John Wiley & Sons, Inc.; 2017.
- Denk H, Abuja, P.M. & Zatloukal, K. Mallory-Denk bodies and hepatocellular senescence: a causal relationship?. Virchows Arch484, 637?644 (2024).
- Doige CE. Omasal squamous cell carcinoma in a ewe. Can J Comp Med 1983;47:382-384.
- Hughes K, Radakovic M, Gorman F, Malinowska B, Cullen JM. Immunohistochemical characterization of clear cell variant of hepatocellular carcinoma in a sheep. J Comp Pathol. 2023 Jul;204:47-50.
- Johnstone AC. Two cases of hepatic mastocytoma in sheep. Vet Pathol 1972;9:159-163.
- Johnstone AC, Manktelow BW. The pathology of spontaneously occurring malignant lymphoma in sheep. Vet Pathol 1978;15:301-312.
- Lovatt FM, Strugnell BW. An observational study involving ewe postmortem examination at a fallen stock collection centre to inform flock health interventions. Vet Rec 2013;172:504.
- Monahan CF, Garner MM, Kiupel M. Hepatocellular Neoplasms in Captive Fennec Foxes (Vulpes zerda). J Zoo Wildl Med 2018;49:996-1001.
- Patnaik AK, Hurvitz AI, Lieberman PH, Johnson GF. Canine hepatocellular carcinoma. Vet Pathol 1981;18:427-438.
- Shih TC, Wang L, Wang HC, Wan YY. Glypican-3: A molecular marker for the detection and treatment of hepatocellular carcinoma?. Liver Res. 2020 Dec;4(4):168-172.
- Zhang W, Wang Q, Jiang YX, et al. Simultaneous double primary clear cell carcinomas of liver and kidney: a case report and review of literature. Int J Clin Exp Pathol 2015;8:995-999.