Wednesday Slide Conference 2022-2023
Conference 15
Case IV:
Signalment:
Adult, female, Sprague Dawley rat, Rattus norvegicus
History:
The animal was in the control group on a 5-week general toxicology study. The animal was dosed with vehicle via oral gavage and had no notable clinical signs.
Gross Pathology:
There were no significant gross lesions.
Laboratory Results:
There were no abnormalities on urinalysis, serum chemistry, or hematology.
Microscopic Description:
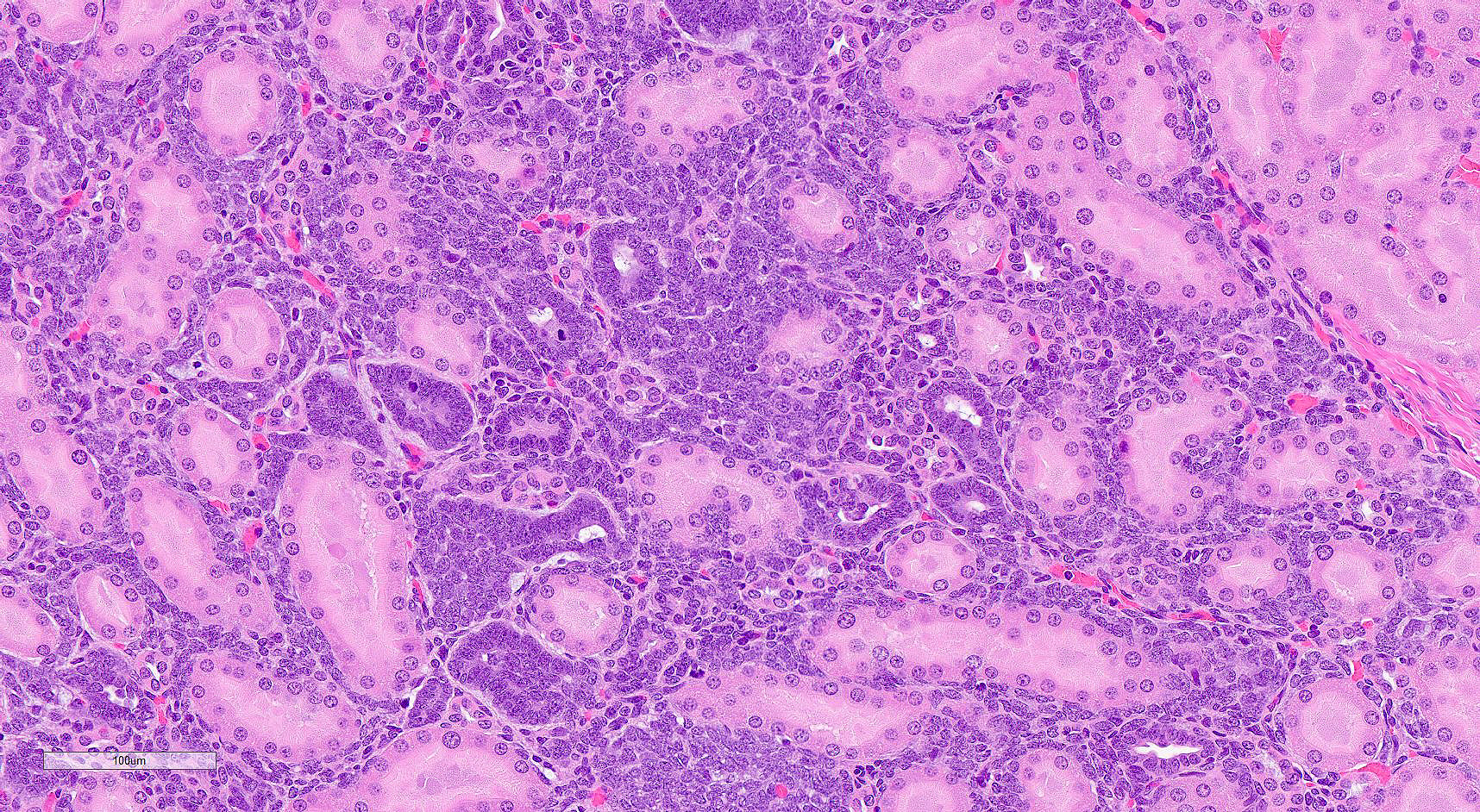
In a section of kidney, at the junction of the medulla and cortex are multifocal areas of proliferative stellate to polygonal blastemal cells separating and infiltrating between normal fully developed tubules supported by a small amount of fine fibrovascular stroma. The blastemal cells have a small amount of pale basophilic cytoplasm, have indistinct cell borders, and are arranged in streams, nests, rosettes, and irregular tubules. The tubules are lined by a single layer of epithelial cells or are piled haphazardly. The cells have ovoid nuclei with finely stippled chromatin and 1-3 nucleoli. There are 1-2 mitoses per high power (400x) field. Small numbers of lymphocytes infiltrate these areas. Adjacent areas of the medulla and cortex appear unaffected.
Contributor’s Morphologic Diagnoses:
Kidney: Multifocal nephroblastematosis
Contributor’s Comment:
Nephroblastematosis is a spontaneous/incidental lesion that can be encountered in rats in toxicologic studies.2 This condition is also known as nephroblastomatosis, blastemal rest, nephrogenic rest, or intralobar nephroblastematosis and has been reported in rats, dogs, and human children.2,5,6,7 Nephroblastematosis can be encountered in any age of rat and may have the potential to develop into nephroblastomas as the lesions enlarge; thus, they are regarded as preneoplastic lesions.1,2,6,7 These lesions are not grossly apparent, and by the time the lesions are large enough to be observed grossly, they are more consistent with a diagnosis of nephroblastoma.7
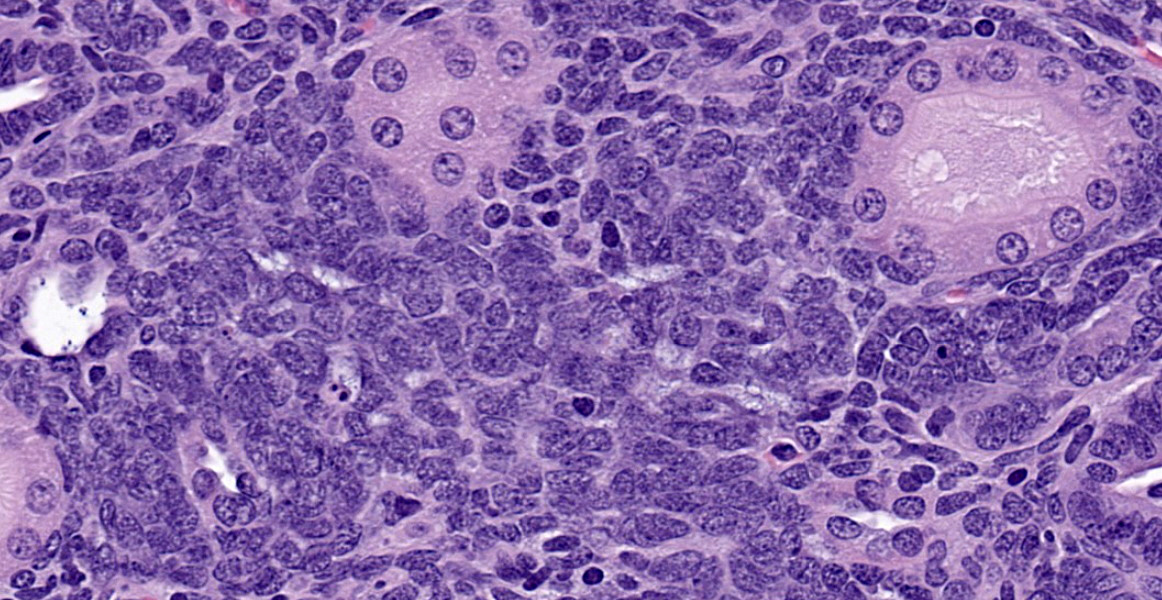
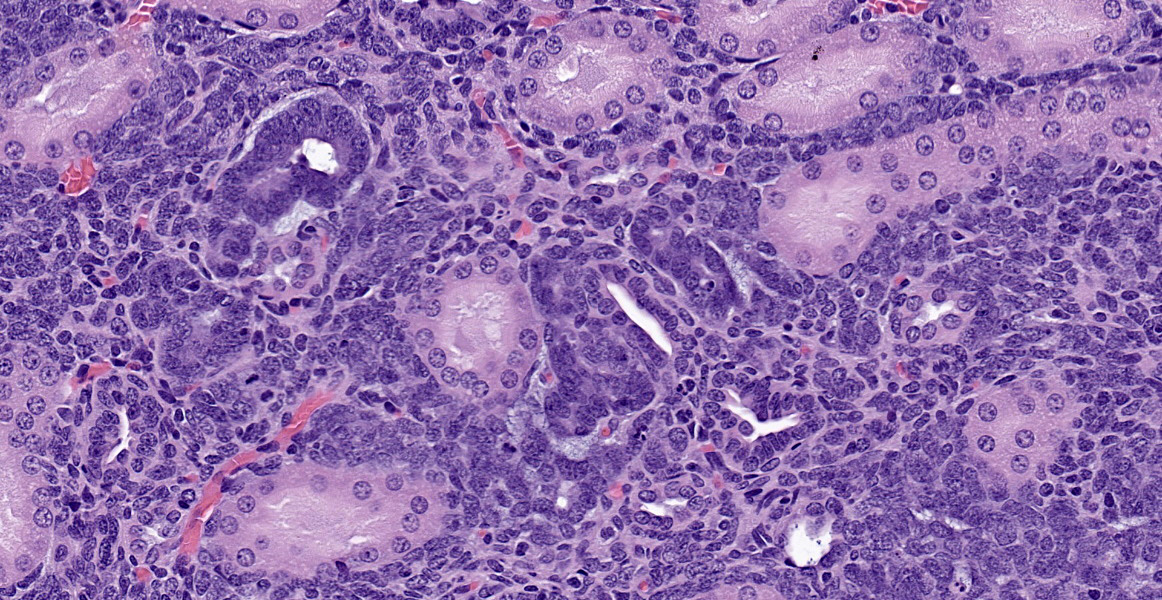
As described, the lesions are composed of microscopic masses at the corticomedullary junction composed of dense blastemal cells with scant basophilic cytoplasm and basophilic nuclei (Figure 1).2 There may be rare renal organoid differentiation into rosettes, glomeruloid structures and tubules (Figure 2).2 Adjacent pars recta tubules may have mitotic figures, which is an autocrine response to the blastema.1 These lesions usually do not cause severe disruption of the surrounding kidney architecture, though may be associated with dilated tubules.7
Nephroblastoma is the main differential for this lesion, though the delineation between these findings appears arbitrary and based on size and degree of organoid differentiation such as more tubules and glomerular structures.2,7 Nephroblastematosis also usually presents with a multifocal nature, as in this case.2
In humans, nephrogenic rests are differentiated into incipient, dormant, involuting, hyperplastic, or neoplastic rests.1,5 Incipient rests have microscopic evidence of proliferation or maturation and are seen in infants.5 Dormant rests are present in older people and may remain unchanged for years.1,5 Involuting, also known as sclerosing or obsolescent, rests are composed of a well-formed tubule lined by a single layer of low-cuboidal epithelium surrounded by dense collagen, and may eventually disappear.1,5 Hyperplastic rests exhibit proliferation in diffuse or focal areas and may progress to grossly apparent masses.1 Neoplastic rests are those in which neoplastic transformation occurs within single cells of the rest which go on to produce Wilms tumor.1 These rests can also be separated into perilobar or intralobar nephrogenic rests.1 Perilobar nephrogenic rests occur at the periphery of the lobe, whereas intralobar rests occur anywhere within the lobe.1 In humans, intralobar nephrogenic rests have been associated with loss or mutation of the WT1 gene.1
Some authors refer to nephroblastematosis in rats, which have a unilobar kidney, as intralobar nephroblastematosis as the blastemal cells occur within the lobule at the corticomedullary junction.7 While some authors consider the terms nephroblastematosis and nephroblastomatosis to be synonymous, others consider the terminology nephroblastomatosis to represent multiple grossly apparent nephroblastomas.2,7 Nephroblastematosis is the preferred terminology by the International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice (INHAND) Project and Standardization for Exchange of Nonclinical Data (SEND).2
Contributing Institution:
Charles River Laboratories, Mattawan, MI
JPC Diagnosis:
Kidney: Nephroblastematosis, multifocal.
JPC Comment:
The contributor provides a thorough description of an uncommonly documented lesion in veterinary species, and this case of nephroblastematosis provides a glimpse of pre-neoplastic changes which may have proceeded the nephroblastoma from Case 3 of this conference.
Nephroblastematosis was first described in a 1961 report of a premature human infant at 32 weeks gestation.4 The cortical surfaces were enlarged and slightly lobulated, and they kidneys were histologically consistent with 14-16-week gestational age, with blastemal cells surrounding areas of stroma, tubules, and glomeruli. 4 In that case, both kidneys were diffusely affected, but it is now known that nephroblastomatosis more commonly occurs focally or multifocally. 4 During normal embryogenesis, migration of the ureteric bud into the metanephric blastema induces development of glomeruli, nephrons, and stroma. 4 Defects earlier in nephrogenesis result in the failure of differentiation in intralobular nephrogenic rests, while later defects result in persistent perilobular rests. 4 Nephroblastematosis occurs when nephrogenic rests persist beyond 34-36 weeks gestation in humans. 4 Nephrogenic rests are found in approximately 1% of all human pediatric autopsies, with perilobar the most common location. 4 All nephrogenic rests have the potential to become neoplastic, either as adenomas (i.e. metanephric adenoma or adenofibroma) or nephroblastomas. 4 In humans, however, regression is much more common, and only approximately 1% progress to neoplasia. 4
As the contributor alludes to, differentiating nephroblastomatosis and nephroblastoma is challenging. In humans, nephrogenic rests generally have irregular margins, lack encapsulation, may have foci of sclerosis between rests, whereas nephroblastomas are generally round with pseudoencapsulation and may lack sclerosis.4 Pre-existing non-neoplastic blastema may be found along the periphery of nephroblastomas.4 Conference participants remarked about the presence of irregular tubules which could lead a pathologist to diagnose nephroblastoma, but elected to follow criteria from human literature described above.
Reports of nephroblastematosis are very rare in veterinary species, and the contributor covers this condition in rats well. Additionally, nephroblastomatosis has also been described as an incidental finding in a single cynomolgus macaque.3
References:
- Beckwith JB. Nephrogenic rests and the pathogenesis of Wilms tumor: Developmental and clinical consideration. Am J Med Genet. 1998;79:268-273.
- Frazier KS, Seely, JC, Hard GC, et al. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Tox Pathol. 2012;40:14S-86S.
- Goens SD, Moore CM, Brasky KM, et al. Neprhoblastomatosis and nephroblastoma in nonhuman primates. J Med Primatol. 2005; 34: 165-170.
- Hennigar RA, O’Shea PA, Grattan-Smith JD. Clinicopathologic features of nephrogenic rests and nephroblastomatosis. Adv Anat Pathol. 2001; 8(5):276-89.
- Jackson CB and Kirkpatrick JB. Nephrogenic rest in a Crl:CD (SD)IGS BR rat. Vet Pathol. 2002;39:588-589.
- Kelaiselvan P, Mathur KY, Pande VV, et al. Intralobar nephroblastematosis in a nine-week-old Wistar rat. Tox Pathol. 2009;37:819-825.
- Mesfin GM. Intralobar nephroblastematosis: Precursor lesions of nephroblastoma in the Sprague-Dawley Rat. Vet Pathol. 1999;36:379-390.