Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 16
29 January 2020
Dr.
Ingeborg Langohr, DVM, PhD, DACVP
Professor
Department of Pathobiological Sciences
Louisiana State University School of Veterinary Medicine
Baton Rouge, LA
CASE IV: N633-16 (JPC 4119040).
Signalment: 4 month-old, female, crossbreed, Sus scrofa domesticus, swine.
History: This animal was raised in a swine farm located in the state of Rio Grande do Sul, Brazil, and was part of an outbreak which occurred in three distinct swine growing-finishing sites. This outbreak affected pigs with age ranging from 80 to 120 days, and it lasted 60 days. The three sites had a total of 2152 pigs, of which 92 died during the outbreaks. All these pigs, including the one submitted, showed clinical signs of apathy, weight loss, diarrhea, cyanosis, and reddening of the ears, abdomen, and distal parts of the thoracic and hind limbs over a clinical course of 7 to 10 days. In addition, approximately 30 animals from the affected group, including the case referred, displayed stiff gait, muscle weakness, hind limb paresis, and recumbency. Euthanasia was elected due to the poor prognosis and progression of clinical signs.
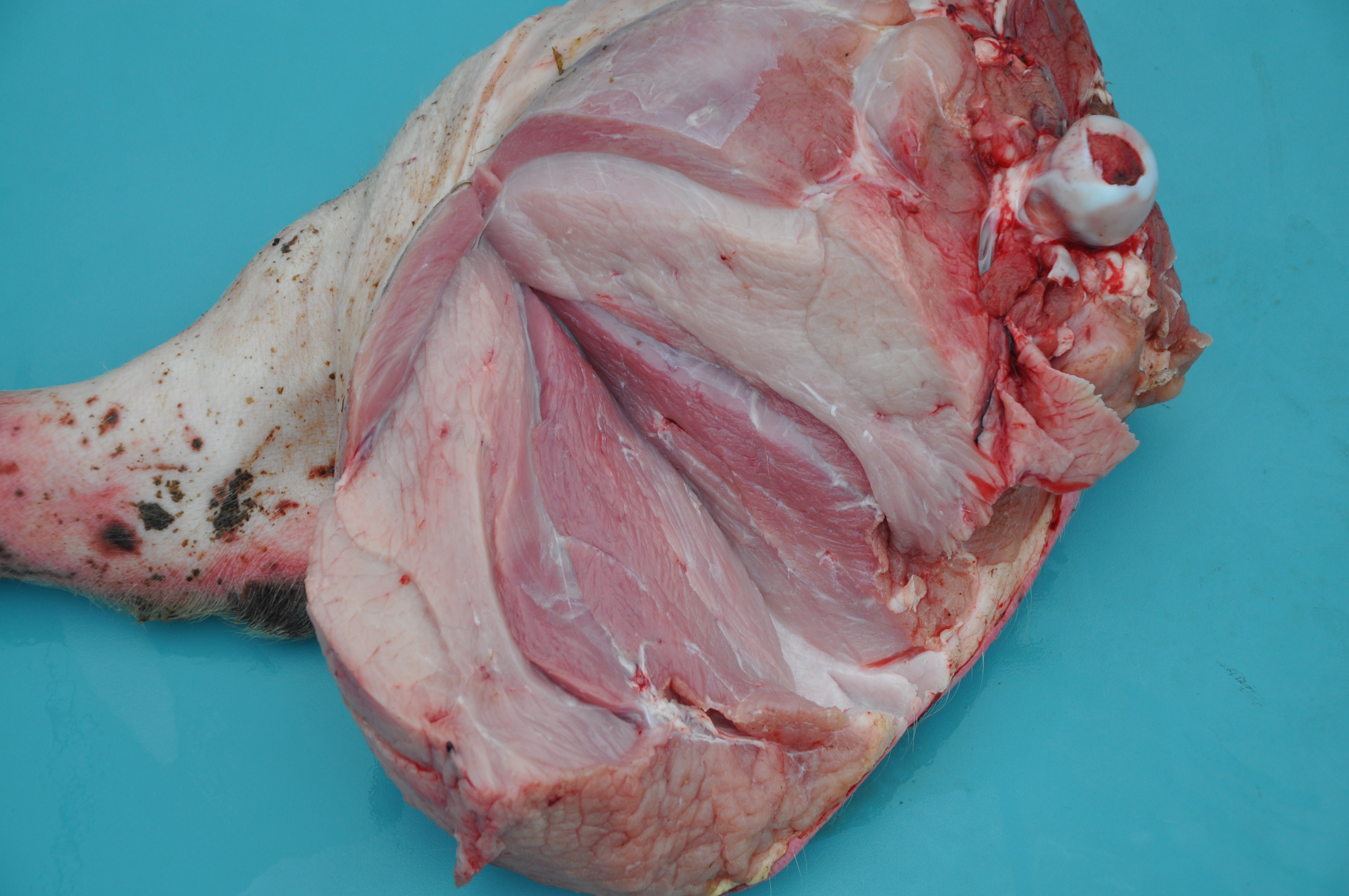
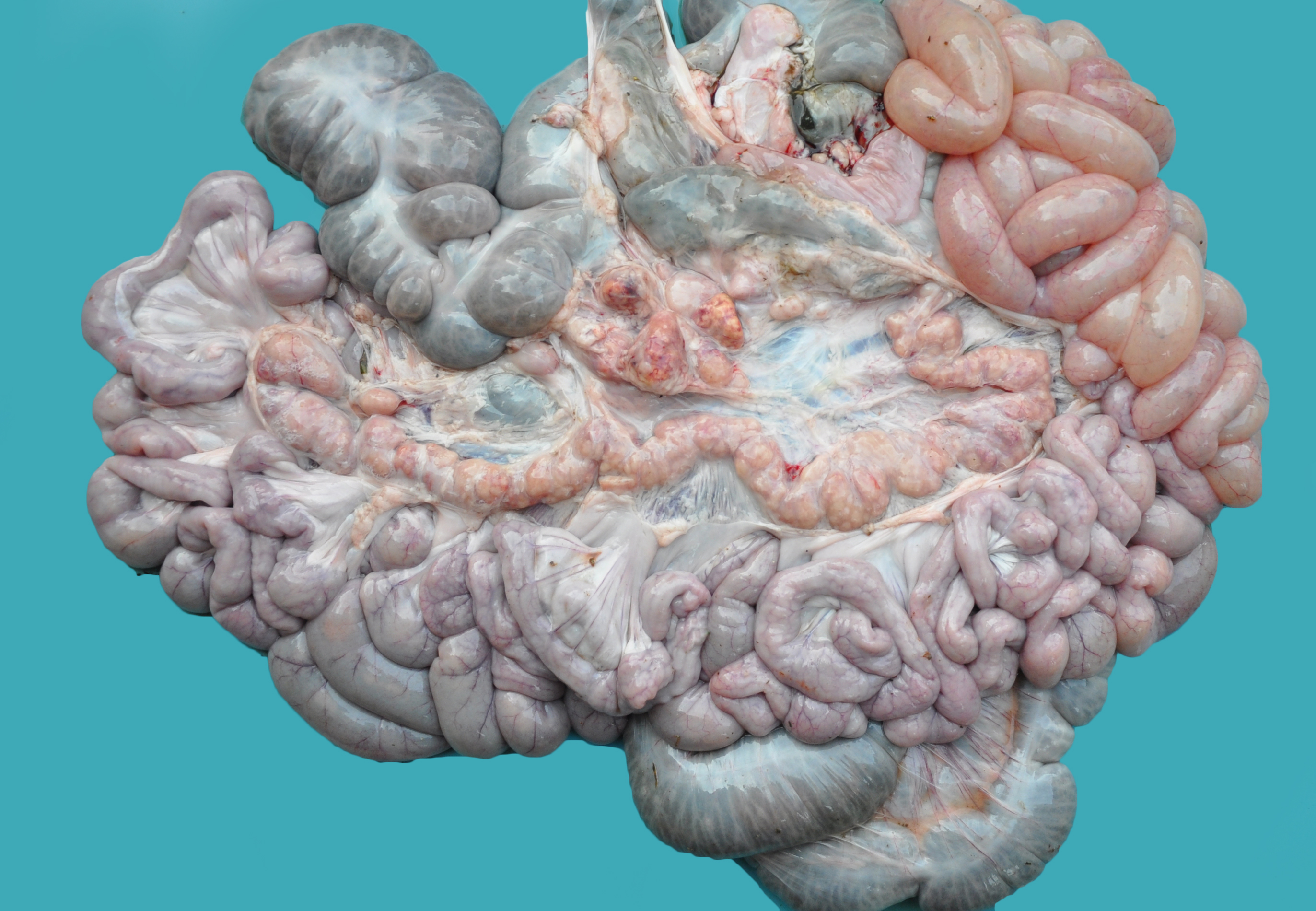
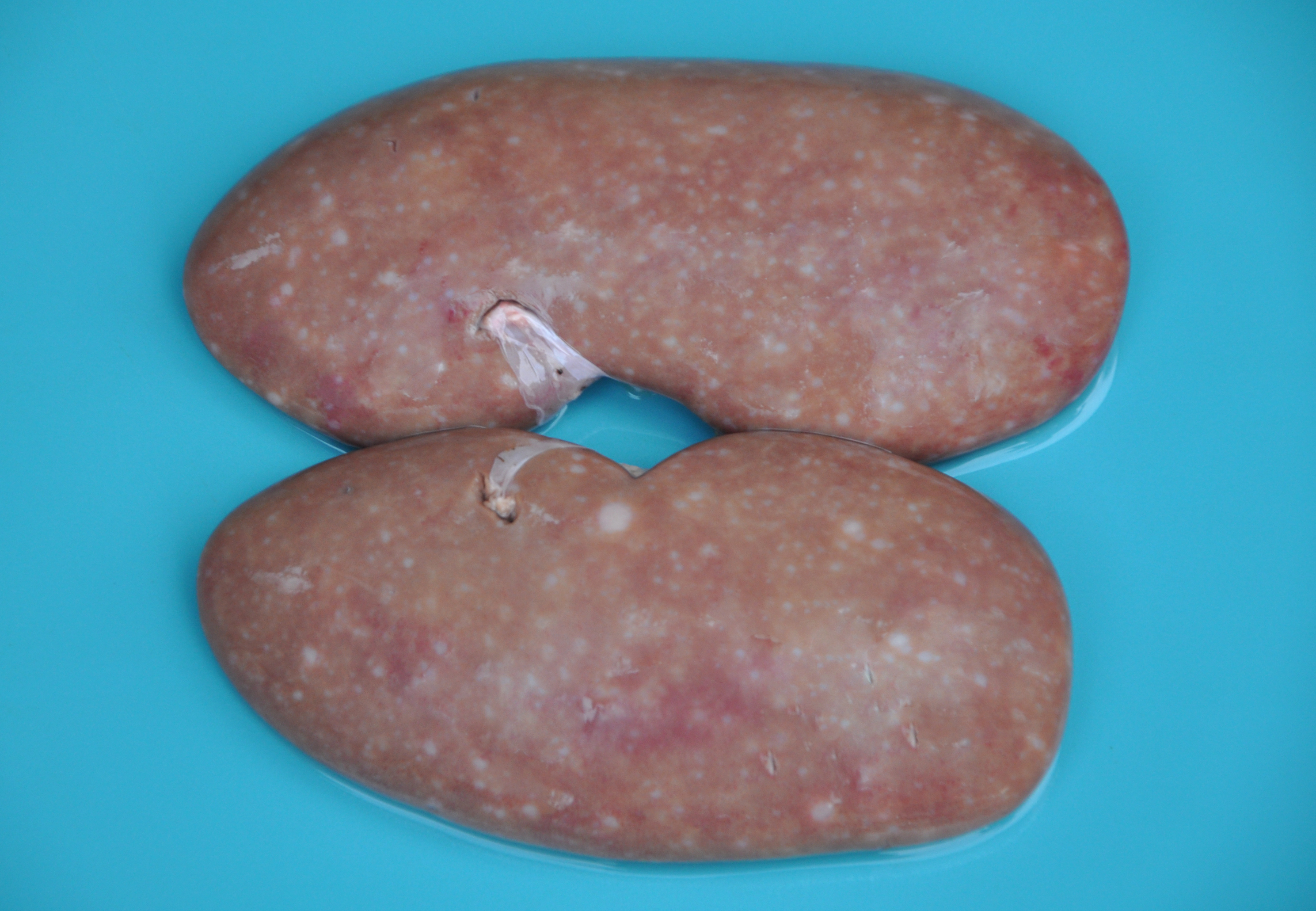
Gross Pathology: Grossly, the muscular lesions consisted of multifocal to coalescing pale discoloration of the skeletal muscles, which was severe for the hind limbs, moderate for the thoracic limbs, and mild for the dorsal lumbar skeletal muscles. Other lesions included generalized lymphadenomegaly with whitish and reddish inter-mixed areas on the cut surface; enlarged kidneys with multifocal to coalescing pinpoint to round whitish areas; and splenomegaly with infarcts and multifocal white pulp hyperplasia. The liver was enlarged with diffuse orange-reddish discoloration; the skin had ulcers and irregular reddened lesions on the limbs and ear cyanosis.
Laboratory results: - Immunohistochemistry (IHC) was performed on lymphoid tissue and was characteristic of PCVD, with intense immunostaining in the cytoplasm of macrophages and multinucleated giant cells and moderate immunostaining of endothelial cells. In addition to that, IHC was performed on skeletal muscle tissue, and it showed intense multifocal immunostaining mainly in the cytoplasm and nuclei of inflammatory cells, while mild immunostaining was evidenced in the cytoplasm of endothelial cells, necrotic fibers, and skeletal muscle satellite cells.
DNA was obtained from the lymph nodes, spleen, and skeletal muscle of this pig, from which a polymerase chain reaction (PCR) product of 481 bp was amplified. DNA extraction and PCR for PCV2 were performed as described previously.4 The PCR products were sequenced, which indicated 99% identity with sequences in GenBank from the PCV2b genotype.
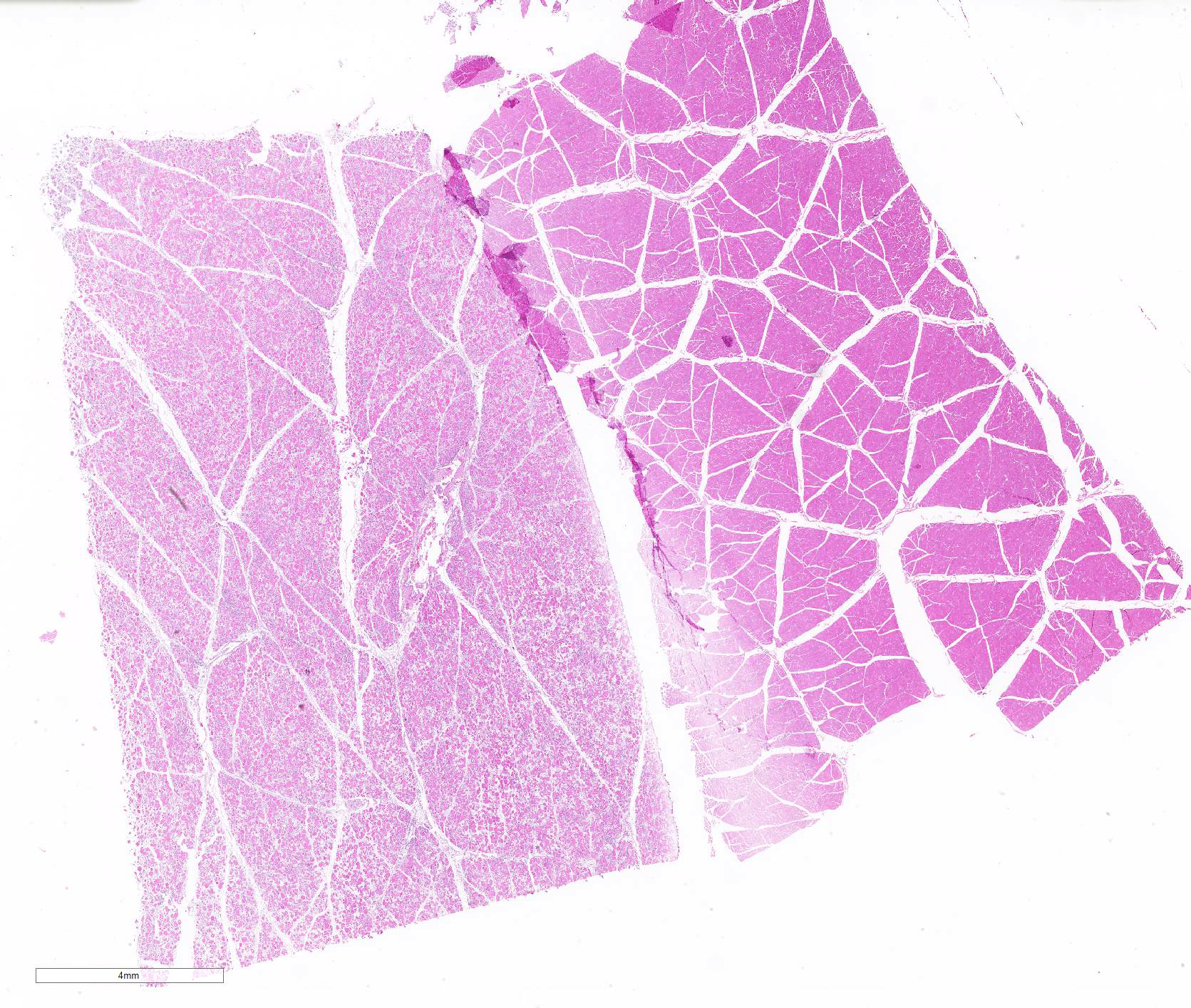
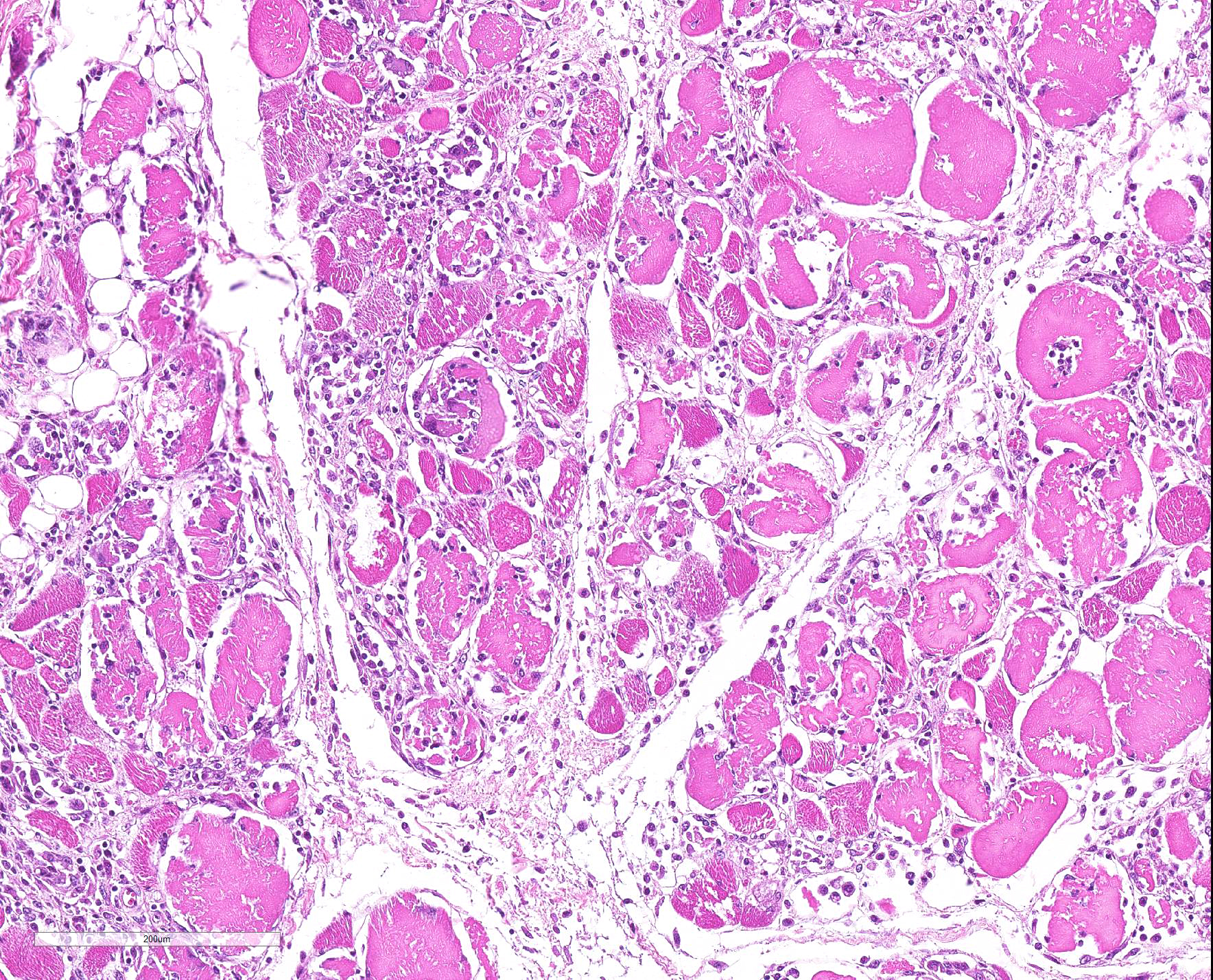
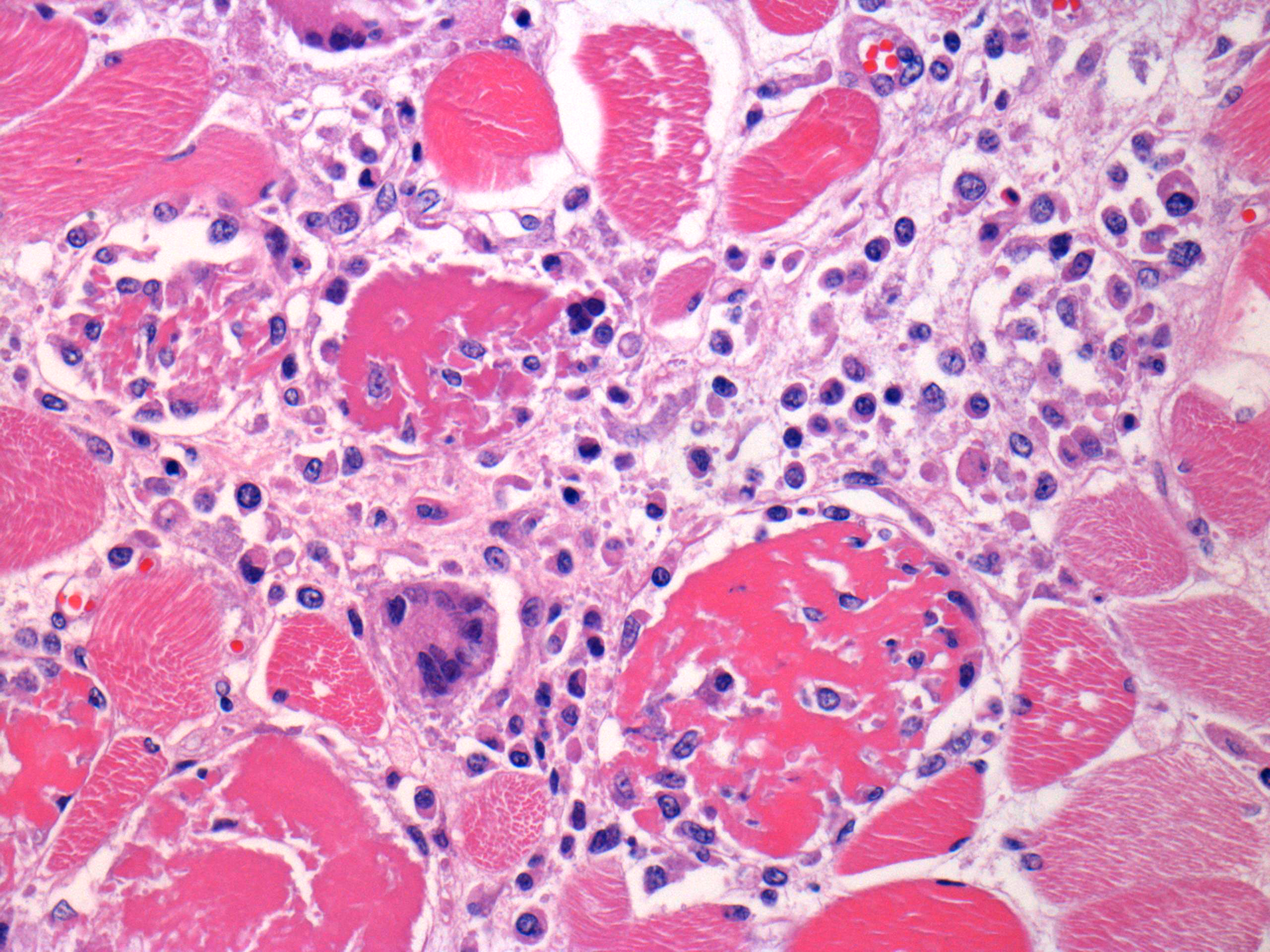
Microscopic Description: Some submitted slides have two to three sections of skeletal muscle, one of which is from the affected animal and presents prominent histological lesions, and the other ones is a section of normal skeletal muscle from an unaffected control pig. The affected tissue section is characterized by multifocal to coalescing, intense hyaline and floccular necrosis of myofibers, which have swollen and hypereosinophilic sarcoplasm with loss of striations, pyknotic nuclei, in addition to myofiber fragmentation with an intense inflammatory infiltrate of macrophages and multinucleated giant cells, sometimes occupying the whole myofiber sarcoplasm. This pig had also in the interstitial and perivascular spaces a moderate inflammatory infiltrate of macrophages, multinucleated giant cells, lymphocytes, and plasma cells, as well as multifocal mild hemorrhage, endothelial cell hypertrophy, mild multifocal thrombosis, as well as occasional regenerating myofibers.
Contributor Morphologic Diagnosis:
Multifocal to coalescing, severe, subacute, granulomatous necrotizing myositis.
Contributor Comment: In the present case, the final diagnosis of granulomatous necrotizing myositis due to porcine circovirus infection was reached through the association of clinical, gross, histopathological, immunohistochemical, and molecular findings. Porcine circovirus type 2 (PCV2) is a nonenveloped, circular, single-stranded DNA virus,17 which presents four genotypes: PCV2a, PCV2b, PCV2c,3,9,15and PCV2d.5,18 The virus is associated with multiple clinical syndromes in pigs, collectively known as porcine circovirus diseases (PCVD).11 Clinically, PCVD may vary, and affected pigs usually display progressive weight loss, dyspnea, diarrhea, and lymphadenopathy. Histologically, lymphoid depletion and infiltration of histiocytes and multinucleated giant cells are observed in lymphoid organs.6 In addition to these organs, PCV2 lesions have been described in the kidneys, small and large intestines, stomach, liver, lungs, central nervous system (CNS), and heart.2,10,11,14
Even though systemic disease is frequently observed associated with such viral infection, skeletal muscle lesions caused by PCV2 had not been described in the scientific literature prior to the present case, which has been recently published.7 The etiology of such particular pathological feature is not completely elucidated. Occasionally, PCV2 infection may induce vascular lesions,,8,11,13 which could induce necrotic changes; however, only mild vascular alterations within skeletal muscle of affected pigs were observed. Thus, we propose that the skeletal muscle lesions observed are most likely a direct effect of the viral infection, since positive immunostaining was noted in the sarcoplasm of myocytes and in macrophages within the muscle.
Based on the gross findings observed in the present case, ionophore toxicity and vitamin E/selenium deficiency should be included as the main differential diagnosis.1,16 Vitamin E/selenium deficiency most commonly affects weaned pigs and, microscopically, causes mineralization of degenerate fibers, whereas ionophore poisoning causes a monophasic and multifocal degeneration of myofibers.1 However, the affected pigs in this outbreak did not consume ionophore antibiotics, and the skeletal muscle microscopic lesions were primarily characterized by granulomatous necrotizing myositis, which causes distinct lesions from the above mentioned toxic and metabolic myopathies.1,16
Contributing Institution:
Faculdade de Veterinária
Universidade Federal do Rio Grande do Sul
Setor de Patologia Veterinária
http://www.ufrgs.br/patologia/
JPC Diagnosis: Skeletal muscle: Myositis, necrotizing and granulomatous, diffuse, severe, with multifocal vasculitis.
JPC
Comment: The
contributor summarizes work that he and a team of investigators published in
the journal Veterinary Pathology in 2018, which was the first published
report of skeletal muscle lesions associated with porcine circovirus-2. The
potential infection of skeletal muscle (in addition to a long list of other
tissues, many of which have appeared in the WSC, as listed below) by porcine
circovirus has been considered for a number of years, as it was demonstrated
that naïve pigs could be infected by feeding skeletal muscle from PCV-2
infected pigs.
In the report by Konradt et al.,7 the granulomatous and necrotizing
myositis seen in these animals was not an isolated manifestation of porcine
circovirus disease, but one of a number of lesions in a group of pigs
afflicted by PCVD. In these animals , lymphoid tissue, including the spleen,
tonsils and lymph nodes, showed marked depletion of lymphocytes and
granulomatous inflammation with giant cells. Multinucleated giant cells appear
to be a fairly constant feature in certain areas of inflammation in PCV-2
infected pigs and were also present in this slide, scattered throughout the
interstitial infiltrate. Affected pigs in this study also
exhibited lymphohistiocytic vasculitis with fibrinoid necrosis in a number of
organs including the skeletal muscle, lymphoid tissue, kidneys, liver, skin,
and leptomeninges. Several pigs additionally exhibited granulomatous
enteritis, predominantly in proximity to Peyer?s patches.7
The moderator mentioned a very important point about granulomatous inflammation in this particular case. While widespread damage to muscle often incites a histiocytic response as part of cleanup, the number of epithelioid macrophages within the interstitium exceeds that which would be expected as a result of muscular necrosis and should be interpreted as granulomatous inflammation.
The moderator closed discussion of this case with a comprehensive review of PCV-2, PCV-2b, and PCV-3.
Since 2007,
porcine circovirus and the many and varied manifestations of porcine
circovirus-associated disease has been an oft-used viral disease and swine
disease in the WSC. While space and time prohibit the recapituation of myriad
presentations of PCVD within this comment, the reader is encouraged to visit
the various cases listed below (used as examples of common manifestations of
PCVD) for a wealth of information on this important swine disease.
The moderator closed discussion of this case with a comprehensive review of
PCV-2, PCV-2b, and PCV-3.
|
Conference 17, Case 2, 2018-2019 Conference 8, Case 2, 2016-2017 |
Granulomatous myocarditis |
|
Conference 5, Case 1, 2017-2018 |
Granulomatous interstitial pneumonia with arteritis |
|
Conference 21, Case 4, 2014-2015 |
Cerebellar hemorrhage |
|
Conference 25, Case 1, 2013-2014 Conference 1, case 4, 2007-2008 |
Granulomatous hepatitis with marked hepatocellular degeneration and loss |
|
Conference 7, Case 3, 2011-2012 |
Granulomatous interstitial nephritis |
|
Conference 8, Case 4, 2009-2010 |
Porcine dermatopathy and nephropathy syndrome |
|
Conference 17, Case 4, 1997-1998 |
Granulomatous lymphadenitis |
References:
1. Cooper BJ, Valentine BA. Muscle and tendon. In: Maxie M, ed. Jubb, Kennedy, and Palmer?s Pathology of Domestic Animals. Edinburgh, UK: Elsevier; 2016: 164?248.
2. Correa AM, Zlotowski P, de Barcellos DE, et al. Brain lesions in pigs affected with postweaningmultisystemic wasting syndrome. J Vet Diagn Invest. 2007; 19(1):109?112.
3. Dupont K, Nielsen ED, Baeko P, et al. Genomic analysis of PCV2 isolates from Danish archives and a current PMWS case-control study supports a shift in genotypes with time. Vet Microbiol. 2008;128(1?2):56?64.
4. Ellis J, Krakowka S, Lairmore M, et al. Reproduction of lesions of postweaning gmultisystemic wasting syndrome in gnotobiotic piglets. J Vet Diagn Invest. 1999;11(1):3?14.
5. Franzo G, Cortey A, Olvera D, et al. Revisiting the taxonomical classification of porcine circovirus type 2 (PCV2): still a real challenge. Virol J. 2015;12(131): 1?8.
6. Kim J, Chae C. Necrotising lymphadenitis associated with porcine circovirus type 2 in pigs. Vet Rec. 2005;156(6):177?178.
7. Konradt G, Cruz RAS, Bassuino DM, et al. Granulomatous Necrotizing Myositis in Swine Affected by Porcine Circovirus Disease. Vet Pathol. 2018; 55(2):268-272.
8. Langohr IM, Stevenson GW, Nelson EA, et al. Vascular lesions in pigs experimentally infected with porcine circovirus type 2 serogroup B. Vet Pathol. 2010; 47(1):140?147.
9. Olvera A, Cortey M, Segalés J. Molecular evolution of porcine circovirus type 2 genomes: phylogeny and clonality. Virology. 2007;357(2):175?185.
10. Opriessnig T, Janke BH, Halbur PG. Cardiovascular lesions in pigs naturally or experimentally infected with porcine circovirus type 2. J Comp Path. 2006; 134(1):105?110.
11. Opriessnig T, Meng XJ, Halbur PG. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest. 2007;19(6):591?615.
12. Opriessnig T, Patterson AR, Meng XJ, Halbur PG. Porcine circovirus Type 2 in muscle and bone marrow is infectious and transmissible to naïve pigs by oral consumption. Vet Microbiol 2009; 133 (1-2), 54-64.
13. Resendes AR, Segalés J. Characterization of vascular lesions in pigs affected by porcine circovirus type 2?systemic disease. Vet Pathol. 2015;52(3):497?504.
14. Segalés J, Allan GM, Domingo M. Porcine circovirus diseases. In: Straw BE, Zimmerman JJ, D?Allaire S, Taylor DJ, eds. Diseases of Swine. 9th ed. Ames, IA: Blackwell; 2006:299?307.
15. Segalés J, Olvera A, Grau-Roma L, et al. PCV-2 genotype definition and nomenclature. Vet Rec. 2008;162:867?868.
16. Shimshoni JA, Britzi M, Pozzi PS, et al. Acute maduramicin toxicosis in pregnant gilts. Food ChemToxicol. 2014;68:283?289.
17. Tischer I, Gelderblom H, Vettermann W, et al. A very small porcine virus with circular single-stranded DNA. Nature. 1982;295(5844):64?66.
18. Xiao CT, Halbur PG, Opriessnig T. Complete genome sequence of a novel porcine circovirus type 2b variant present in cases of vaccine failures in the United States. J Virol. 2012;86(22):12469?12473.