Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 3
4 September, 2019
Dr. Corrie Brown, DVM, PhD, Diplomate ACVP
Josiah
Meigs Distinguished Teaching Professor
University Professor
Department
of Pathology
University of Georgia College of Veterinary Medicine
2200 College Station Road Athens, GA, 30602
CASE III: 2019B (JPC 4134828).
Signalment: Pig, Sus scrofa domesticus, LWD, 8-weeks-old, female
History: This is one of two experimental cases of pigs infected with classical swine fever virus (CSFV). This case was intraorally inoculated with 1mL of 106.5TCID50/mL of a strain of CSFV. Clinical signs of the animal were observed and total leukocytes in the whole blood of the pig were counted daily for 2 weeks. Clinical samples of the heparinized hole blood, sera, saliva, nasal swab and feces were collected for the detection of the virus gene by RT-PCR analysis.
The pigs inoculated with CSFV showed fever and abolition of appetite from 4 days post infection (dpi) and developed reddened skin lesion in hindlegs and conjunctivitis at 14 dpi. The pig was euthanized at 14 dpi.
Gross Pathology: In the pig infected with CSFV, multifocal infarction of the margin of the spleen was observed. Mild hyperemia on the brain was seen in the pig. Hemorrhagic lymph nodes in the pig were appeared. Button ulcers in the colon was find in the pig. There were no pathological findings in the respiratory organs in the pig.
Laboratory results: The body temperature of this CSFV inoculated pig was over 40oC from 4 to 14 dpi. The decrease of leukocyte counts in blood (under 10,000 cells/μl) were also confirmed from 3 to 14 dpi. The viral RNA was detected from the blood samples from 4 dpi and from saliva, nasal swab and feces from 5 dpi in the pig.
Microscopic Description:
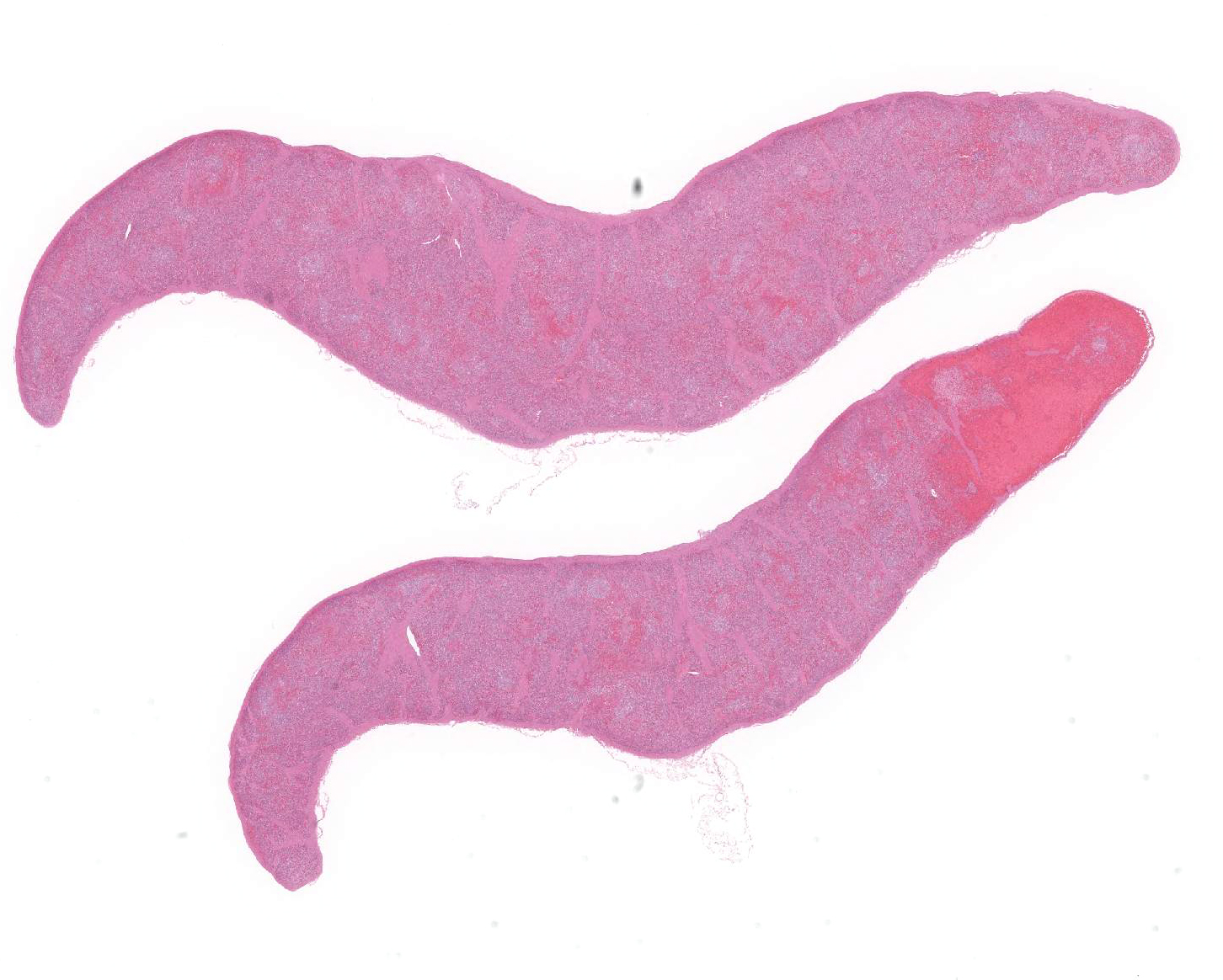
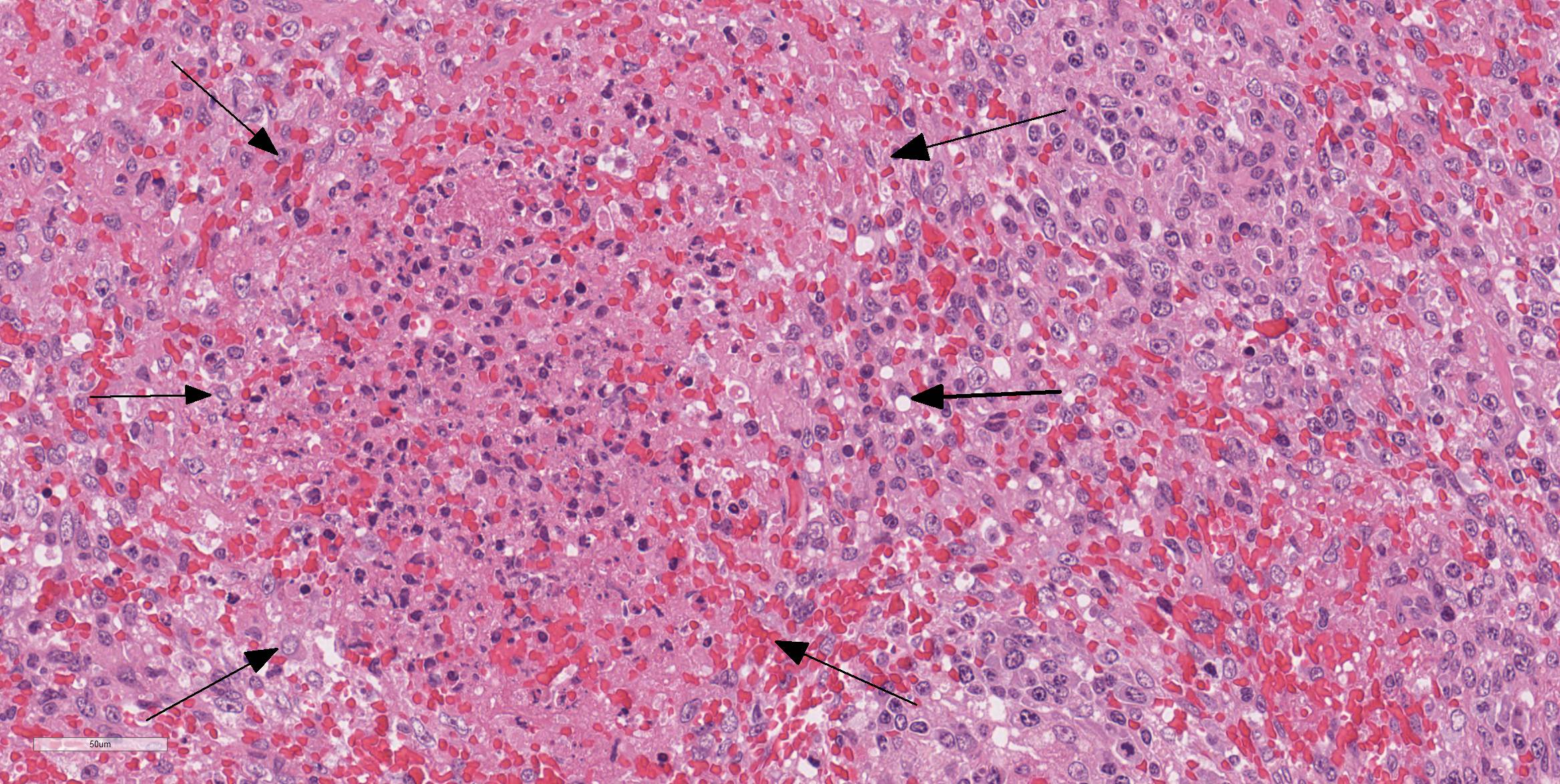
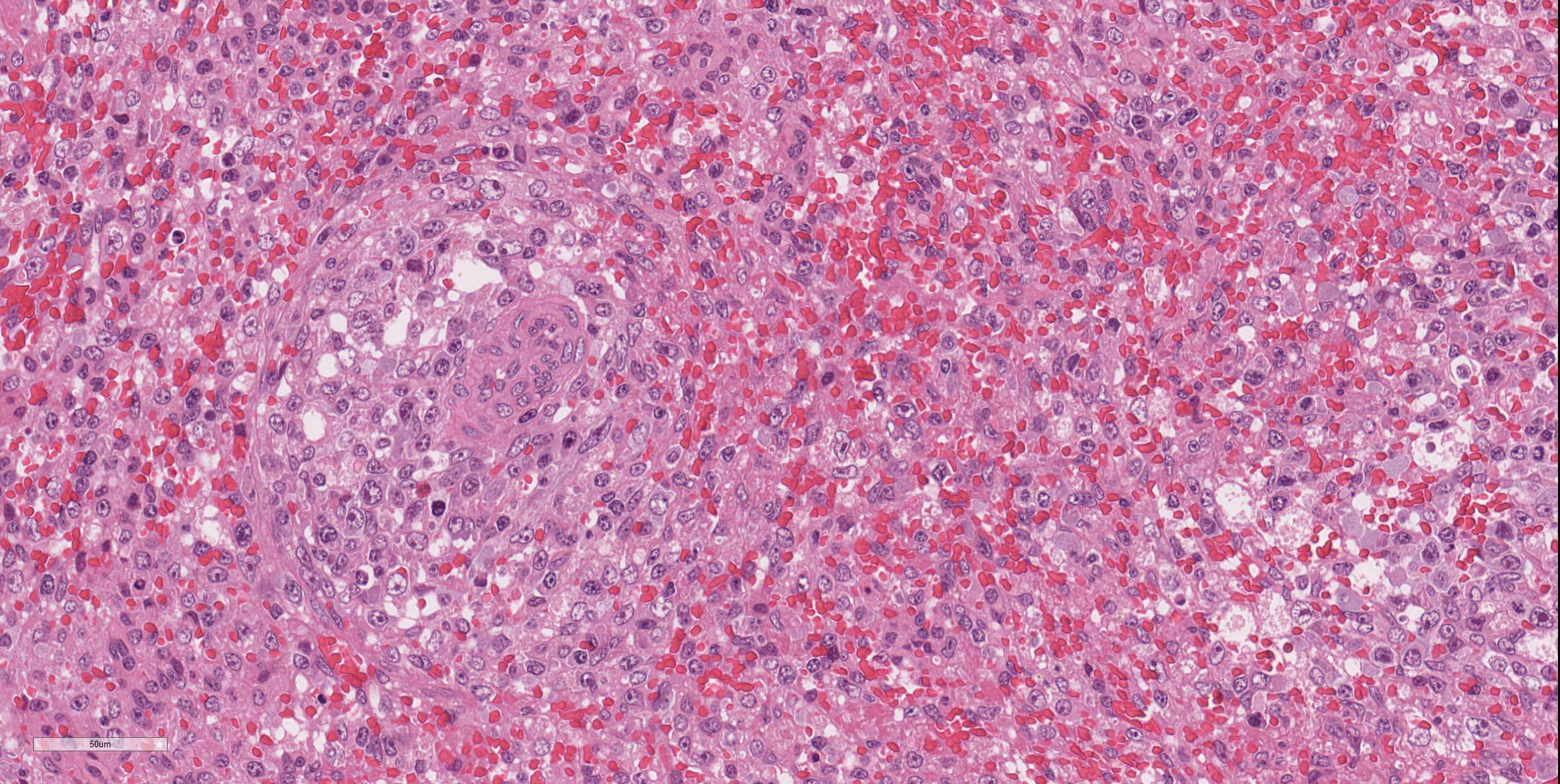
Spleen: There were multifocal necrosis with hemorrhage, karyopyknosis and karyorrhexis in the red pulp. The lumen of some blood vessels was dilated and was occluded by a fibrin thrombus. Necrosis of arterial walls were occasionally observed; however, vasculitis was not prominent. The stromal and parenchymal cells in the wedge-shaped margin of the spleen was replaced by extravascular erythrocyte accumulation. The remaining stromal cells in that area showed necrosis with karyopyknosis and karyorrhexis (hemorrhagic infarction).
The white pulp in the adjacent parenchyma is of markedly reduced cellularity (lymphoid depletion) and almost of lymph follicles were atrophic.
Contributor Morphologic Diagnosis:
1. Spleen, red pulp: Multiple fibrin thrombosis with hemorrhagic infarction, multifocal necrosis, moderate.
2. Spleen, white pulp: Lymphocyte depression, diffuse, severe.
Contributor Comment: We confirmed that the characteristic lesion of multifocal infarction of the margin of the spleen arises following inoculation with CSFV by intraoral inoculation. The histological lesion of hemorrhagic infarction was reproduced experimentally; however, vasculitis was not prominent in this case, suggesting this case was in an early stage of an infection of CSFV.
Classical swine fever (CSF) is a highly contagious viral disease induced by CSFV of the genus Pestivirus in the family Flaviviridae.3 This disease is widely distributed in the world including Asian countries. CSFV is classified into three genotypes (1, 2 and 3) and several subgenotypes (1.1-1.4, 2.1-2.3, and 3.1-3.4).4,7 In East and Southeast Asia, several genotypes or subgenotypes of CSFV isolates including 1.1, 2.1-2.3, and 3.4 have been identified.1,5 The virulence of the CSFV ranges from highly virulent with almost 100% mortality, to avirulent. The severity also depends on the condition of host animals including age, breeds and health status.2
In the present experiment, the infected pigs developed clinical signs of lethargy, anorexia, reddish dermal macula and conjunctivitis. These clinical symptoms were quite mild, and the pig did not die within 2 weeks of the experimental period. These results suggest that the virulence of the CSFV used in this study is considerably lower than a high virulent strain of CSFV.2
Splenic infarction is considered pathognomonic for CSF.2 Macrophage-driven cytokine storms following infection of CSFV play an important role of the pathogenesis of splenic infarctions in CSF. 2 A marked increase in macrophage/monocyte-derived pro-inflammatory cytokines such as TNFα, IL-1, and IL-6 is suggested to be the main mediator of systemic endotheliotoxicity. These cytokines induce endothelial swelling, followed by degeneration and necrosis of endothelial cells and fibrinonecrotizing vasculitis.13 The formation of arterial infarcts at the multifocal sites of vasculitis within the spleen leads to vascular occlusion followed by ischemic necrotic cell death of the dependent parenchyma. A single splenic arterial supply with minimal anastomoses is an important predisposing factor for infarction in the spleen.6 This infarction area is rapidly followed by hemorrhage from damaged blood vessels and inflow of erythrocytes from the surrounding parenchyma with intact perfusion leading to the commonly illustrated hemorrhagic infarcts.6
Severe lymphocyte depression is also common in the splenic white pulp of the CFSV-infected pigs. In acute stages of infection, CSF is accompanied by severe lymphopenia and resulting immunosuppression as well as granulocytopenia.2,8,11 The infection of dendritic cells induces secretion of large amount of IFN-α which is a key element in the pathogenesis of CSF. The high serum level of IFN-α is suggested to be the central inducer of dysregulation of the immune system, which manifests as lymphocyte apoptosis, depletion and immunosuppression.10
The acute form of the disease is characterized by atypical clinical signs such as high fever, anorexia, gastrointestinal symptoms, general weakness and conjunctivitis during the first two weeks upon infection of CSFV and then skin lesions including hemorrhages, cyanosis or reddish macula appeared in different areas of the body such as the ear, limbs and ventral abdomen around two to four weeks after infection.2,9 In this study, this typical skin lesion could find from 14 dpi in the inoculated pig and the clinical appearance was quite difficult to diagnose as CSF in this experimental study. The results in this study showed that the pigs infected with this strain of CSFV excrete viruses into the clinical samples and viral RNAs were detected from the blood samples, especially whole blood samples, from 4 or 5 dpi until at least 2 weeks after infection. Because it is difficult to detect clinical sigms in pigs infected with this strain, the results in this study are significantly valuable to establish countermeasures and diagnostic strategy for CSFVs that has similar characteristics with mild pathogenesis.
Contributing Institution:
3-1-5 Kannondai, Tsukuba, Ibaraki 3050856,Japan
National Institute of Animal Health
http://www.naro.affrc.go.jp/english/laboratory/niah/
JPC Diagnosis: 1. Spleen, margins:
Necrosis, multifocal, moderate, with thrombosis, hemorrhage, and fibrin deposition
(infarcts).
2. Spleen, white pulp: Lymphoid depletion, diffuse, severe.
JPC Comment: The contributor has done a nice job describing an investigation of the effects of intraoral inoculation of a mildly virulent strain of classical swine fever virus (CSFV). CSFV, a pestivirus in the same family as bovine viral diarrhea virus and border disease virus, is one of the most economically important viruses in the world. While a number of countries have eliminated the virus (United States, Canada, Mexico and many Western European countries), the virus remains endemic in many others, including China, India and many countries in Central and South America. A number of countries that had eliminated CSF have recently seen recurrences in light of a large current outbreak in China.15
The disease
of classical swine fever is divided into acute and chronic forms. The acute
form is also known as the "lethal" form (although both forms end with death of
infected pigs) or the "transient" form (another odd choice of name.) In the
acute form, clinical signs appear after 4-7 days, and non-specific (referred to
as "atypical" signs) clinical signs of fever, pyrexia and gastrointestinal
distress are the rule.2 From 2-4 weeks, more diagnostic signs of
including neurologic deficits, paralysis and convulsions appear, and are often
accompanied by cutaneous hemorrhage and cyanosis of the extremities and abdomen
(the so-called "typical" of classic signs of CSF. Due to the immunosuppression
which accompanies CSF (such as seen in the section of spleen submitted in this
case), respiratory and gastrointestinal disease may also be present. Infection
of pregnant sows may result in abortions, stillbirths, and mummification.2
Chronic infections are usually associated with immunotolerant pigs who were
infected during gestation. These infections, while prolonged and mostly
associated with "atypical" signs, ultimately also result in death of infected
animals; albeit with a longer course of several months in which they
continually shed high levels of virus in the saliva, urine, feces and other
secretions. Death is
often the result of immunosuppression, and affected animals exhibit marked
depletion of lymphoid organs and ulceration of the gastrointestinal tract.2
CSF control is based largely on two strategies - comprehensive culling (which
is effective in countries which have eliminated the virus), and vaccination,
which is practiced in countries in which the CSFV is endemic.15 While
many of these countries now mandate vaccination against CSV, small and medium
pig farms (in which help irregular vaccination schedules or the maintenance
of immune-tolerant animals) may help to maintain the virus in an endemic
state. Vaccination failure may be further impacted by the presence of multiple
genotypes in a given area or immunosuppressive coinfections by other viruses
include PRRS virus and PCV-2. In some countries, the virus is maintained in
the population of wild boars from which it may spill over into the domestic pig
population, especially on small farms.15
Another potential weapon in the fight to eradicate CSV in endemic areas is the development of virus-resistant animals. Recently, Chinese scientists reported the development of genetically modified pigs using CRISPR-Caspase 9 mediated knock-in technology. Antiviral small hairpin RNAS were inserted into porcine DNA at the Rosa26 locus and transgenic pigs were produced by somatic nuclear transfer. F1-generation pigs were challenged with virulent virus and and developed only minor clinical symptoms and markedly decreased blood levels of virus as compared to non-transgenic controls.12
One of the more interesting aspects of this particular slide is the relatively mild changes noted in the splenic arterioles, which rather than a significant vasculitis (which would be expected given the endotheliotropism often exhibited by this virus) exhibited at best a mild vasculopathy with no mural inflammation and rare pyknosis of mural smooth muscle cells and a hint of protein within the wall. A spirited discussion of the usage of the terms vasculitis and vasculopathy for this change in the absence of any significant inflammation failed to yield a clear victor.
To further illustrate the marked lymphoid depletion in this specimen, a JPC-run CD20 highlighted the almost absolute lack of B-cells (a favorite target of CSFV), and a CD3 showed marked depletion of T-cells. An IBA-1 stain showed numerous macrophages, many of which appeared activated.
References: 1. Beer M, Goller KV,
Staubach C, Blome S. Genetic variability and distribution of Classical swine
fever virus. Anim Health Res Rev 2015:16(1):33â39. 2. Blome S, Staubach C,
Henke J, Carlson J, Beer M. Classical swine fever-an updated review. Viruses
2017; 9(4) E86
doi:10.3390/v9040086. 3. Kirkland PD, Le
Potier MF, Vannier P, Finlaison D. Pestiviruses. In: Zimmerman, J. J.,
Karriker, L. A., Ramirez, A., Schwartz, K. J. and Stevenson, G. W. eds. Diseases
of swine. 10th ed. West Sussex: John Wiley & Sons; 2012; 538â553. 4. Lowings P, Ibata G,
Needham J, Paton D. Classical swine fever virus diversity and evolution. J
Gen Virol 1996; 77(Pt6):1311â1321. 5. Luo Y, Li S, Sun Y,
Qiu HJ. Classical swine fever in China: a minireview. Vet Microbiol 2014; 172(1-2):1â6. 6. Mosier DA. Vascular
Disorders and Thrombosis. In: Zachary JF, ed. Pathologic Basis of Veterinary
Disease. 6th ed. St. Louis, Missouri: Elsevier; 2017; 44-72. 7. Paton DJ, McGoldrick
A, Greiser-Wilke I, Parchariyanon S, Song JY, Liou PP, Stadejek T, Lowings JP,
Björklund H, Belák S. Genetic typing of classical swine fever virus. Vet
Microbiol 2000; 73(2-3):137â157. 8. Pauly T, König M,
Thiel HJ, Saalmüller A. Infection with classical swine fever virus: Effects on
phenotype
and
immune responsiveness of porcine T lymphocytes. J Gen Virol 1998; 79(Pt1):31â40. 9. Petrov A, Blohm U,
Beer M, Pietschmann J, Blome S. Comparative analyses of host responses upon
infection with moderately virulent classical swine fever virus in domestic pigs
and wild boar. Virol J 2014; 11:134 doi:
10.1186/1743-422X-11-134. 10. Summerfield A, Ruggli
N. Immune responses against classical swine fever virus: between ignorance and
lunacy. Front Vet Sci 2015; 2-10. 11. Susa M, König M,
Saalmüller A, Reddehase MJ, Thiel HJ. Pathogenesis of classical swine fever: B-lymphocyte
deficiency caused by hog cholera virus. J Virol 1992; 66(2):1171â1175. 12. Xie Z, Pang D, Yuan
H et al. Genetically modified pigs are protected from classical swine fever
virus. PLoS 1 Pathog 2018; 14(2), e1007193. 13. Zachary JF.
Mechanisms of microbial infections. In: Zachary JF, ed. Pathologic Basis of
Veterinary Disease. 6th ed. St. Louis, Missouri: Elsevier;
2017; 132-241. 14. Zhou B. Classical
swine fever in China â an update minireview. Front Vet Sci 2019; 6:187,
10.3389/fvets.2019.00187.