CASE 1: 19-0026A (JPC 4139463-00)
Signalment:
4 year old female entire Ragdoll (Felis catus)
History:
Ileocecocolic mass with severely enlarged lymph nodes. Preliminary diagnosis of feline eosinophilic sclerosing fibroplasia based on eosinophils seen on tissue smear. Associated lymph nodes markedly enlarged and purulent discharge present on incision. Ileocecocolic mass resected along with enlarged purulent lymph nodes for histopathology. DDx feline eosinophilic sclerosing fibroplasia vs neoplasia (mast cell tumor, lymphoma, adenocarcinoma, other).
Gross Pathology:
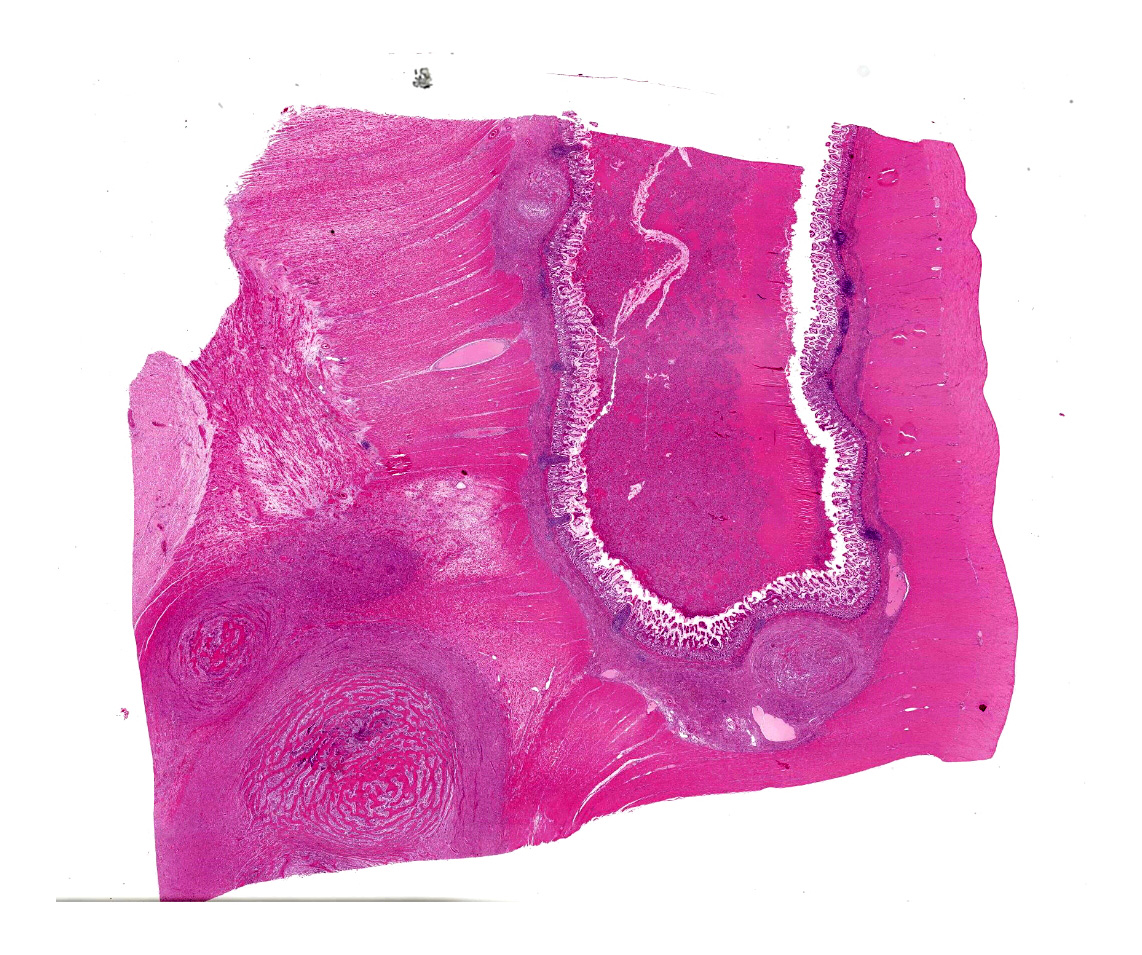
The specimen presented for examination is a section of the ileum, caecum and colon and a mesenteric lymph node. A firm, pale tan-cream, multilobulated mass 120 x 40 x 12 mm is effacing the caecum, ileum and colon. On section, the mass contains soft pale brown-green material surrounded by a thick, cream, firm capsule. The mesenteric lymph node (65 x 58 x 55 mm) is firm, multilobulated, cream-pale tan. On section, the lymph node contains multiple cystic structures which exude viscous brown fluid.
Laboratory results:
RAPID DIFF SMEAR
Mass: Much connective tissue and numerous spindle cells, scattered neutrophils, eosinophils and macrophages, numerous bacterial cocci.
Lymph node: Spindle cells, several neutrophils, scattered macrophages, occasional plasma cell; some cocci are present in neutrophils and the occasional bacterial rod may also be present.
CULTURE (Blood agar, aerobic, anaerobic 37C)
All samples (mass, discharge and node) grew a heavy growth of Staphylococcus aureus. The sample labeled "lymph node" additionally grew a heavy mixed bacterial population that includes obligate anaerobes.
SENSITIVITY
Susceptible to oxacillin, amoxycillin/clavulanic acid, 1st generation cephalosporins, doxycycline, trimethoprim / sulfonamides, marbofloxacin, enrofloxacin, gentamicin, clindamycin, erythromycin.
Resistant to penicillin, ampicillin.
Microscopic description:
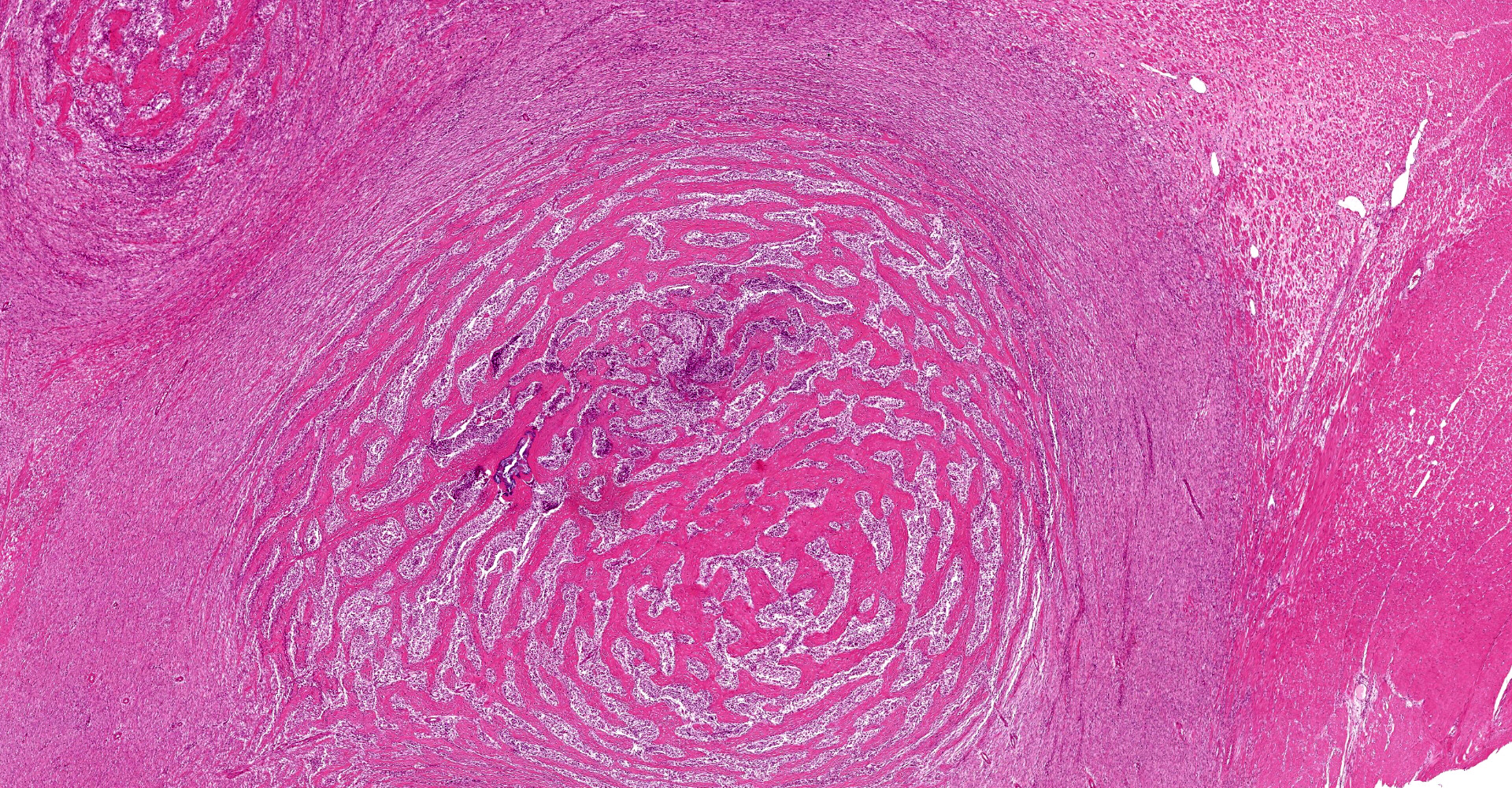
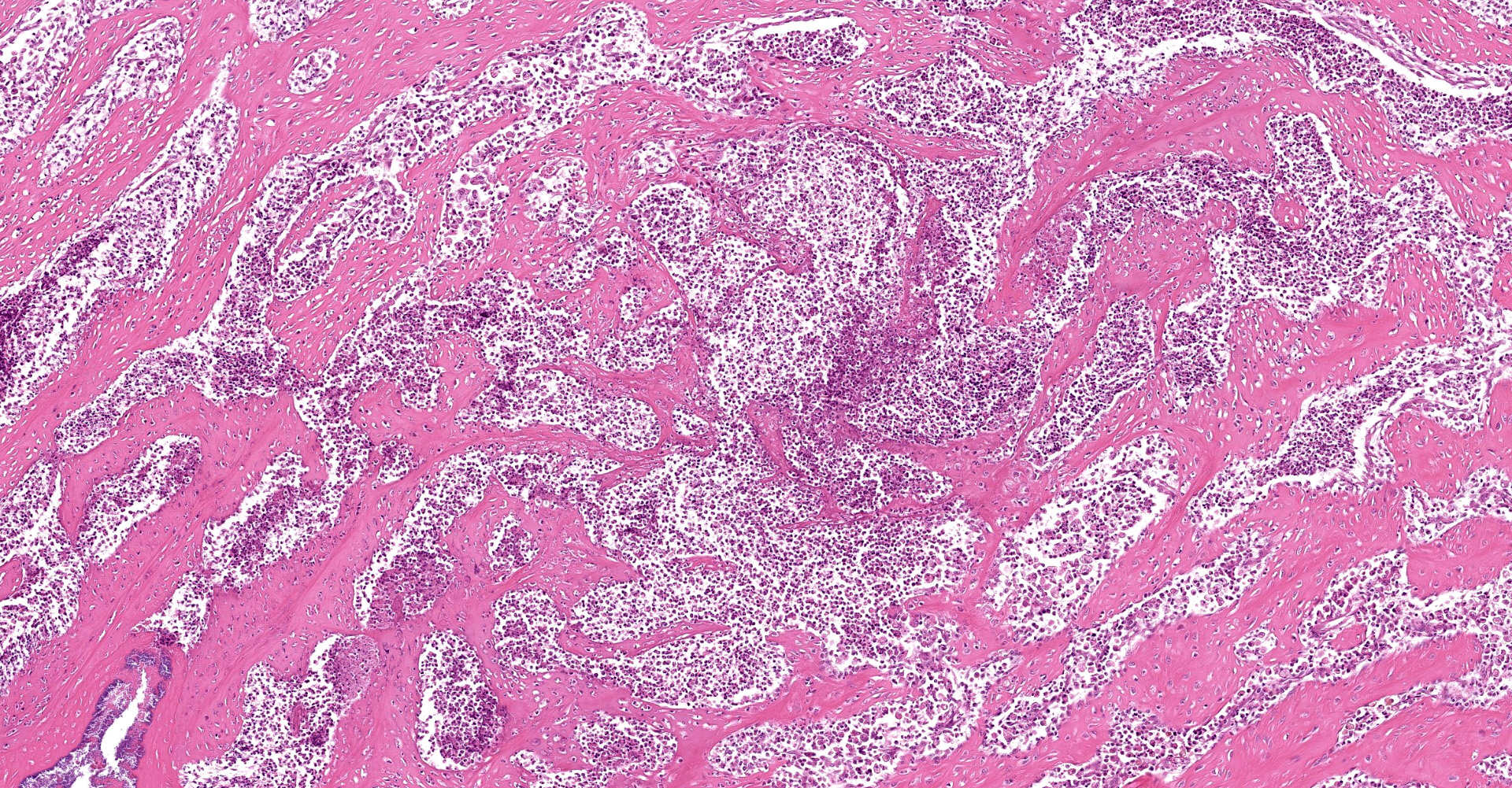
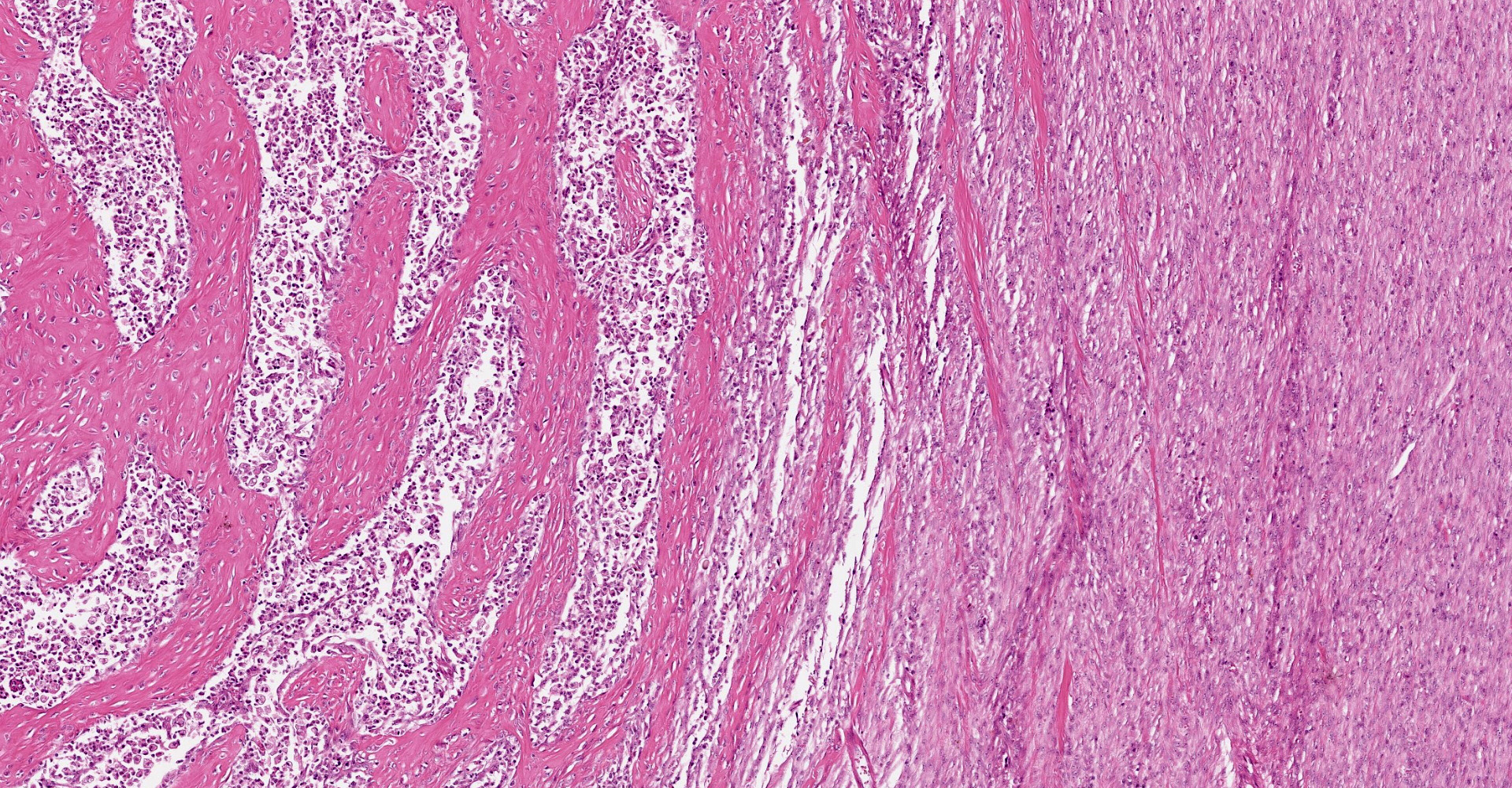
A multifocal proliferative mass composed of thick branching and anastomosing, poorly cellular trabeculae of dense birefringent collagen and plump (reactive) fibroblasts (fibrosis) admixed with abundant degenerate and nondegenerate eosinophils and brightly eosinophilic granular material is expanding and effacing the submucosa, tunica muscularis, serosa and surrounding fibroadipose tissue. The trabecular collagen merges gradually into more typical granulation tissue at the periphery of the lesions. Fewer foamy macrophages, neutrophils and lymphocytes admixed with variably sized foci of cellular and karyorrhectic debris (necrosis), oedema and hemorrhage are scattered throughout the mass. There are rare clusters of cocci. Blood vessels are congested. Numerous lymphocytes, eosinophils and plasma cells admixed with mild oedema and increased amounts of pale collagen and plump (reactive) fibroblasts are infiltrating the lamina propria and extending transmurally through the ileal and colonic wall. The lumen contains cellular and karyorrhectic debris (necrosis) admixed with clusters of basophilic cocci. Occasional ectatic crypts lined by attenuated epithelium contain cellular and karyorrhectic debris (necrosis). There is mild multifocal crypt epithelial hyperplasia, characterized by plump, crowded epithelial cells which pile up, often have overlapping nuclei with frequent mitotic figures. The predominantly eosinophilic inflammation and fibroplasia extend to the colonic surgical margin.
Contributor's morphologic diagnosis:
Ileocecocolic junction, lymph node (not submitted): Eosinophilic sclerosing fibroplasia, with large intralesional colonies of cocci and Splendore-Hoeppli material.
Intestines: Enterocolitis, lymphoplasmacytic, eosinophilic, locally extensive, moderate, chronic, with fibrosis and crypt hyperplasia.
Contributor's comment:
Feline gastrointestinal eosinophilic sclerosing fibroplasia (FGESF) is a feline inflammatory condition which affects the gastrointestinal tract and associated lymph nodes. It is characterized by an intramural, typically ulcerated, multinodular, fibrosing and eosinophilic mass that effaces the wall of the gastrointestinal tract, most commonly at the pyloric sphincter or ileocecocolic junction.2 The disease primarily affects middle-aged cats, although cats of any age can be affected. FGESF has been reported in domestic shorthairs, Ragdolls, Persians, domestic longhairs, mixed breeds, one Siamese, one Maine Coon, and one Himalayan.2,8,10 Ragdolls were overrepresented in a review of 13 cases.8 Typical clinical signs include decreased appetite, vomiting, weight loss and diarrhea.8 A hard, non-painful abdominal mass is often palpable clinically. During sampling, masses are often described as firm, "gritty", and heterogeneous on section.5,8 Bloodwork abnormalities associated with the disease include hyperglobulinemia, hypoalbuminemia, peripheral eosinophilia and/or mild peripheral neutrophilia.2,8,11
The pathogenesis of FGESF is incompletely understood. It is thought immune dysregulation (genetic predispositions, parasitism, dysbiosis, inflammatory bowel disease or food hypersensitivities leading to eosinophil dysregulation), infections (herpesvirus, bacteria, fungi, Toxoplasma gondii, Cylicospirura spp.), and/or migrating foreign bodies may play a role.4,5,8,9 Secondary infections may also perpetuate the inflammatory response. In cases where intralesional bacteria have been detected (as in this case), it is hypothesized that bacteria could initiate FGESF. However, the role of bacteria is still unclear, and the presence of normal bacterial flora further complicates establishment of an association.2,8,11 It is likely pathogenesis is multifactorial. The profound inflammatory response noted in FGESF may be related to other eosinophilic diseases in cats (eosinophilic granuloma, indolent ulcer, eosinophilic plaque).
Eosinophils play a major role in the pathogenesis of FGESF. Eosinophils are most commonly associated with parasitic and allergic conditions. They have several types of granules, including small granules, primary granules, and large specific granules (secondary granules). These granules contain various enzymes, cytokines and proteins that contribute to ongoing inflammation and fibrosis. Specifically, granules contain mediators such as major basic protein (MBP), TGF-? and -?, and Il-1 to IL-6. Eosinophil chemoattractants include histamine, eosinophilic chemotactic factor A, C5a, cytokines (IL-4, IL-5, and IL-13), and chemokines (CCL-5, also known as RANTES, and CCL-11, also known as eotaxin). These chemoattractants are released from epithelial cells, helminths, mast cells, and eosinophils themselves.2,8,10,11 This chronic inflammation likely contributes to eosinophilic proliferation and fibrosis, resulting in mass formation.
Microscopically, FGESF has a characteristic pattern of densely collagenous and anastomosing trabeculae, large spindle cells (myofibroblasts)2 and numerous eosinophils.2,8,10,11 Lesions infiltrate the intestinal wall and, sometimes, regional lymph nodes. There is frequent intestinal mucosal ulceration within affected areas.
The prognosis is guarded and dependent on the ability to completely surgically resect the mass (lesions associated with the pyloric sphincter are unlikely to be completely resected).2,4,11 There is no consistent treatment regime.8 A multimodal treatment approach combining surgical resection, prednisolone, antibiotics, and immunomodulatory agents is considered ideal.8
Differential diagnoses in cats with abdominal mass(es) and/or mesenteric lymphadenopathy include neoplasia (fibrosarcoma, extraskeletal osteosarcoma, feline sclerosing mast cell tumor, lymphoma, adenocarcinoma), granuloma, and eosinophilic gastritis. Clinically, the presence of an ulcerated mass at the pyloric sphincter or ileocecocolic junction can be misdiagnosed as a neoplasm or granuloma. Microscopically, FGESF needs to be distinguished from other neoplasms, including extraskeletal osteosarcoma, where densely collagenous trabeculae may be misinterpreted as osteoid, and feline sclerosing mast cell tumors, due to the presence of low numbers of well-differentiated, perivascular mast cells admixed with numerous eosinophils.6
Contributing Institution:
University of Sydney
JPC diagnosis:
Ileum: Enteritis, pyogranulomatous and eosinophilic, moderate, chronic, multifocal to coalescing, with sclerosing fibroplasia and colonies of cocci.
JPC comment:
The contributor provides a thorough summary of this infrequent disease in cats. As the contributor notes, many cases have been potentially associated with bacteria or fungi, but their role in the development of this disease remains unknown. This disease has a variety of presentations, including a recent case of FGESF confined to the mesentery in a mixed breed cat. The mesenteric nodules were not resectable, but the cat responded to a combination of prednisolone and ciclosporin A. At the time of the report, remission of disease had been achieved, with peripheral eosinophilic resolved, at 689 days post diagnosis. While the pathogenesis is shrouded in mystery, immunomodulatory treatments appear to be efficacious.7
While the mesenteric case of FGESF stands alone, a second recently reported case shares a feature that is not typically described: cavitation. A mural lesion located in the proximal duodenum and incorporating the left limb of the pancreas was multilobulated, with pockets of pale colored semi-liquid material that was gritty and contained some solid concretions, consistent with an abscess.3
In human literature, and to a lesser degree veterinary literature, describe IgG4-related diseases, with a specific subset of sclerosing diseases. The topic was briefly discussed in Conference 3 this year in regard to sclerosing angiomatoid nodular transformation (SANT) of the spleen. IgG4 is the least common of the four IgG subtypes, does not activate complement, and is a Th2-dependent isotype. IgG4 may also act as a protective, blocking antibody in allergen induced IgE-mediated effector cell activation in parasitic infections. A common presentation in human literature includes a mass lesion in the pancreas, with prominent lymphoplasmacytic inflammation, and abundant fibrosis. These cases usually have elevated serum IgG4 levels, with resulting lesions sometimes causing obstructive jaundice. Similar to feline FGESF, this human disease responds well to steroid therapy.1 Cats with FGESF may have elevated IgG4 levels, but that parameter has not been reported. This may represent an avenue for additional research.
The moderator emphasized that the most common differentials for a gastrointestinal mural mass would include this entity, FGESF, as well as feline intestinal sclerosing mast cell tumor, gastrointestinal carcinoma, sarcomas, and lymphomas. Within the set of gastrointestinal lymphomas, the most common subtypes in cats include enteropathy-associated T-cell lymphoma (EATL) type I and II, diffuse large B-cell lymphoma (DLBCL), and large granular lymphocyte lymphoma (LGL).
References:
1. Cheuk W, Chan JKC. IgG4-related Sclerosing Disease: A Critical Appraisal of an Evolving Clinicopathologic Entity. Adv Anat Pathol. 2010;17(5): 303-332.
2. Craig LE, Hardam EE, Hertzke DM, Flatland B, Rohrbach BW, Moore RR. Feline gastrointestinal eosinophilic sclerosing fibroplasia. Veterinary pathology. 2009;46: 63-70.
3. Davidson GA, Taylor SS, Dobromylskyj MJ, Gemignani F, Renfrew H. A case of an intramural, cavitated feline gastrointestinal eosinophilic sclerosing fibroplasia of the cranial abdomen in a domestic longhair cat. J Feline Medicine and Surgery. 2021;7(1). doi:10.1177/2055116921995396.
4. Eckstrand CD, Barr BC, Woods LW, Spangler T, Murphy B. Nematode-associated intramural alimentary nodules in pumas are histologically similar to gastrointestinal eosinophilic sclerosing fibroplasia of domestic cats. Journal of comparative pathology. 2013;148: 405-409.
5. Grau-Roma L, Galindo-Cardiel I, Isidoro-Ayza M, Fernandez M, Majo N. A case of feline gastrointestinal eosinophilic sclerosing fibroplasia associated with phycomycetes. Journal of comparative pathology. 2014;151: 318-321.
6. Halsey CH, Powers BE, Kamstock DA. Feline intestinal sclerosing mast cell tumour: 50 cases (1997-2008). Veterinary and comparative oncology. 2010;8: 72-79.
7. Kambe N, Okabe R, Osada H, et al. A case of feline gastrointestinal eosinophilic sclerosing fibroplasia limited to the mesentery. Journal of Small Animal Practice. 2020;61:64-67.
8. Linton M, Nimmo JS, Norris JM, et al. Feline gastrointestinal eosinophilic sclerosing fibroplasia: 13 cases and review of an emerging clinical entity. Journal of feline medicine and surgery. 2015;17: 392-404.
9. Ozaki K, Yamagami T, Nomura K, Haritani M, Tsutsumi Y, Narama I. Abscess-forming inflammatory granulation tissue with Gram-positive cocci and prominent eosinophil infiltration in cats: possible infection of methicillin-resistant Staphylococcus. Veterinary pathology. 2003;40: 283-287.
10. Suzuki M, Onchi M, Ozaki M. A case of feline gastrointestinal eosinophilic sclerosing fibroplasia. Journal of toxicologic pathology. 2013;26: 51-53.
11. Weissman A, Penninck D, Webster C, Hecht S, Keating J, Craig LE. Ultrasonographic and clinicopathological features of feline gastrointestinal eosinophilic sclerosing fibroplasia in four cats. Journal of feline medicine and surgery. 2013;15: 148-154.