CASE II:
Signalment:
~1 day old neonatal calf; no sex provided; Angus-cross; bovine (Bos taurus)
History:
A rancher found a dead neonatal calf. It was the seventh neonatal death that year among 108 calves. The reported vaccination history was that dams received three products. These were a modified live polyvalent product containing BoHV-1, BRSV, BVDV and parainfluenza 3 virus; Campylobacter fetus bacterin; and a polyvalent inactivated product containing bovine rotavirus, bovine coronavirus, Clostridium perfringens type C, and Escherichia coli. No information was available about recent illness in the dam cohort.
Gross Pathology:
A veterinary practitioner did the necropsy. The laboratory received fresh and fixed tissues. No placental tissue was submitted.
Laboratory Results:
<10 ppm nitrate in aqueous humor (strip test)(within reference range); light growth of E. coli and β-hemolytic Staphylococcus sp. (lung; liver; small intestine; large intestine)(aerobic culture); Ureaplasma sp. detected (lung)(PCR); Clostridium perfringens type A, small intestine (anaerobic culture/PCR).
Contributor's Morphologic Diagnoses:
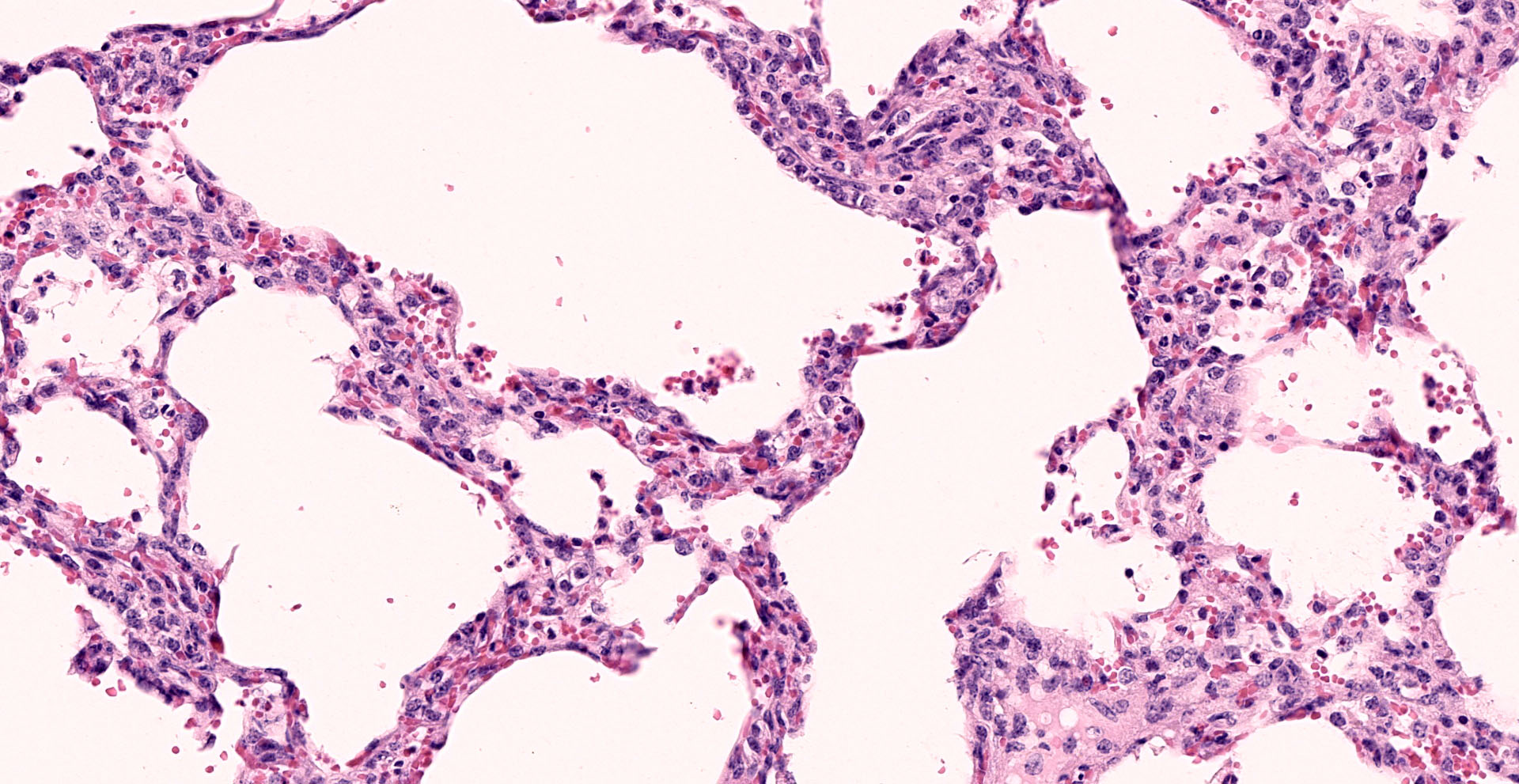
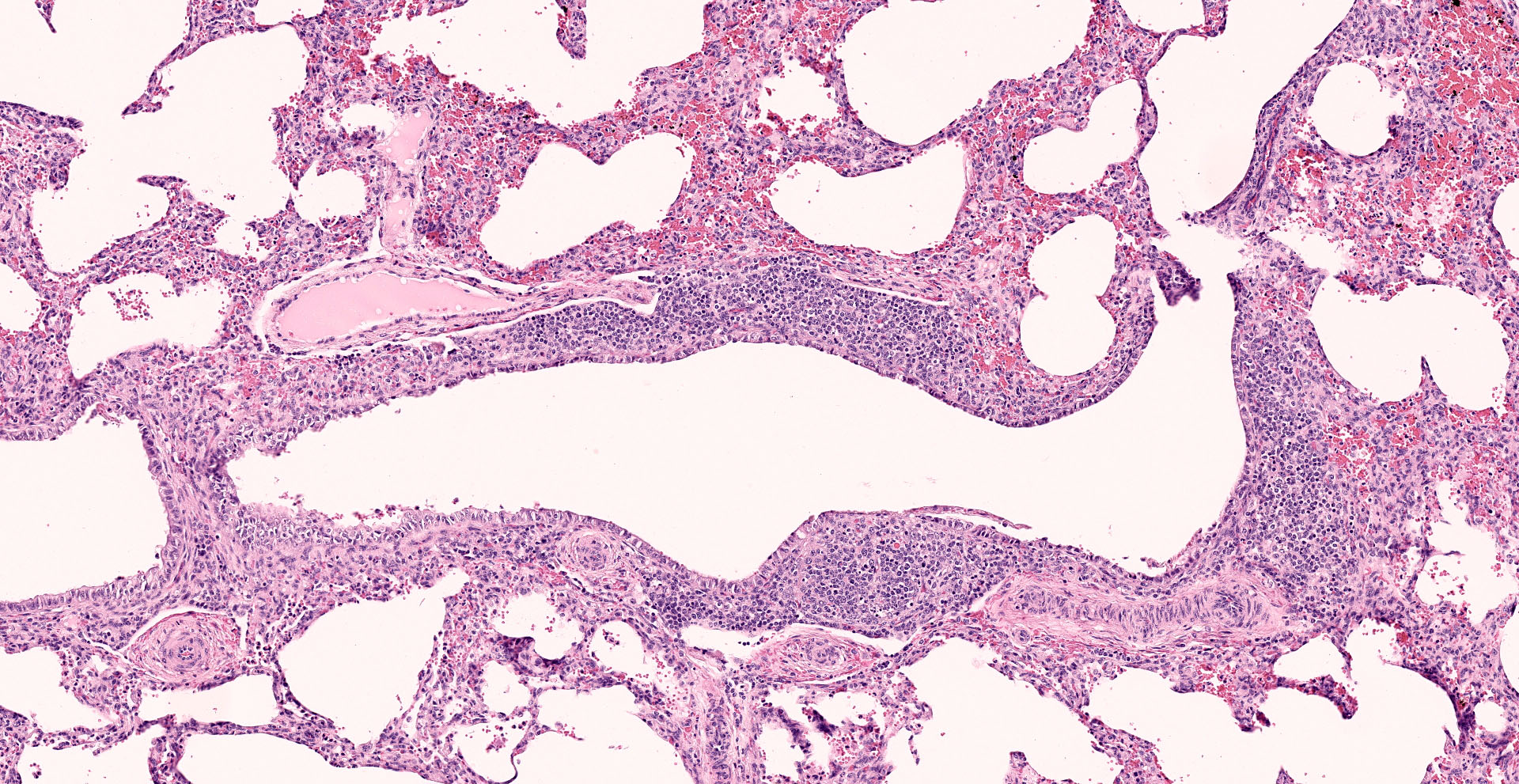
Lung: Interstitial pneumonia, mild, diffuse, with lymphoid hyperplasia of bronchus- and bronchiolar-associated lymphoid aggregates (BALT).
Contributor's Comment:
Other features in the submitted tissue are multifocal intra-alveolar hemorrhage, fibrin
exudation in BALT, and histiocytic alveolitis. The detection by PCR of Ureaplasma diversum is significant, given the character of the pulmonary changes. Our laboratory did not receive some of the other tissues that help support a diagnosis of ureaplasmosis. Non-pulmonary lesions can include chorioamnionitis, erosive conjunctivitis with prominent goblet cells, and erosive arthritis.4,7-9 Gross pulmonary lesions in bovine fetuses that are aborted due to ureaplasmosis are subtle or absent (firm, poorly aerated), and rarely recognized at necropsy. Most laboratory-diagnosed cases of abortion due to U. diversum occur in the third trimester. Some calves, as in this case, go to term and die shortly thereafter. Abortions can occur earlier in gestation. U. diversum also causes embryonic loss, although its importance is unclear.9
Our bacteriology laboratory tests lungs of aborted bovine fetuses for U. diversum by PCR whenever lesions suggestive of ureaplasmosis are found. Hallmark features are diffuse interstitial pneumonia and BALT hyperplasia, often in a minimally autolytic fetal carcass. Most such cases test positive by PCR. Placental and fetal pulmonary lesions have been reproduced experimentally following intra-amniotic challenge of cattle.7 Amnionic lesions comprising mutifocal necrosis with fibrosis and hemorrhage are said to be characteristic. According to one experienced diagnostic pathologist, U. diversum abortion in cattle is infrequent in the United States.2 It is apparently more common in eastern and western Canada. Published surveys of bovine reproductive wastage found that ureaplasmosis accounted for 2.8% and 4.3% of losses.3 Slaughterhouse surveys indicate that U. diversum is rarely found in fetuses and placentas from healthy cattle.
In the past, laboratory diagnosis of U. diversum infections relied on culture. Successful isolation necessitated rapid transport of samples to the laboratory, specialized media, and expertise in ureaplasma culture and identification.8 This is now largely supplanted by PCR using various published molecular protocols.14 Controversy and uncertainty attend many
aspects of ureaplasmosis in cattle due to the occurrence of multiple serotypes, the suspected existence of pathogenic and non-pathogenic strains, and failure of some experimental infections to induce disease.1
Ureaplasma are members of the family Mycoplasmataceae, order Mycoplasmatales. They are so named because they possess urease activity.5 In the past they were called T-mycoplasmas, and presumed to be non-pathogenic. U. diversum is a common commensal in the reproductive tract of male and female cattle. It also colonizes nasal passages. U. diversum is considered one cause of bovine granular vulvitis syndrome. Other, less common conditions in cattle are endometritis, salpingitis, bovine seminal vesiculitis, and infertility. U. diversum is a common contaminant of bull semen, where it survives freezing for artificial insemination. Abortions due to Mycoplasma and Ureaplasma infection are sporadic with few accounts of herd outbreaks.14 Based on experimental studies using caesarean-delivered, colostrum-deprived calves, U. diversum is also a low-grade pulmonary pathogen. It induces atelectasis, mild interstitial pneumonia, peribronchiolar lymphoid cuffs, and BALT hyperplasia.11
On a comparative note, related agents U. urealyticum and U. parvum are isolated from human amniotic fluid and placentas where they are associated with adverse pregnancy outcomes. These include preterm births, neonatal death, chorioamnionitis, low birth weight, pneumonia, and chronic lung disease.12,13
Contributing Institution:
Wyoming State Veterinary Laboratory; 1174 Snowy Range Road; Laramie; Wyoming 82070. http://www.uwyo.edu/wyovet/
JPC Diagnosis:
Lung: Pneumonia, interstitial, histiocytic, moderate, diffuse, with marked BALT hyperplasia.
JPC Comment:
The contributor provides a concise review of ureaplasmosis, an infrequent cause of reproductive failure in cattle associated with the key histologic features of interstitial pneumonia and BALT hyperplasia.
Ureaplasma is one of several genera within Mollicutes, a class composed wall-less bacteria from which their name is derived (mollis and cutis; Latin for "soft" and "skin", respectively). Composed of 14 genera and approximately 200 species, Mollicutes are considered to be the smallest self-replicating free-living organisms and are widely found in plants, animals, and humans. Mollicutes evolved from a Gram-positive precursor though a process known as genomic reduction, resulting in the loss of non-essential genes, such as those encoding the peptidoglycan cell wall and variable metabolic functions. Some Mollicutes are able metabolize carbohydrates (i.e. glucose fermenters) or amino acids such as L-arginine for energy via the arginine dehydrolase pathway. However, these pathways are defunct in other Mollicutes such as Ureaplasma spp. Thus Mollicutes metabolism is often tailored to specific microenvironments, which explains the narrow host ranges and niches of many species. Mollicutes that inhabit animals as commensals, saprophytes, or pathogens fall into three genera: Mycoplasma, Ureaplasma, and Acholeplasma.10
Interestingly, ATP production in Ureaplasma spp. occurs via an alternative metabolic pathway dependent on the hydrolysis of urea into ammonia via urease. This results in an intracellular accumulation of ammonia/ammonium and the creation of an electrochemical gradient utilized to synthesize ATP via the combined actions of an ammonia transporter and a FOF1-ATPase, both of which are bound to the trilaminated cell membrane. In addition, ureaplasmas also generate ammonia via degradation of L-histidine through the action of L-histidine ammonialyase. Regardless of its initial source, the released ammonia results in local irritation to mucous membranes in the infected urogenital and respiratory tract.10 In addition to the local production of ammonia, other virulence factors of Ureaplasma spp. include the ability to adhere to and invade host cells, a range of lipid-associated membrane protein (LAMP) compounds, and the modulation of both prostaglandin synthesis and apoptosis.10
Endometrial cells infected with Ureaplasma spp. exhibit a significant reduction in the synthesis of prostaglandins E2 and F2a from arachidonic acid. This phenomenon is mediated by Ureaplasma spp. membrane phospholipases, such as phospholipase D. Given prostaglandins play a significant role in bovine embryo implantation and pregnancy maintenance, this modulation likely plays a major role in U. diversum's association with premature bovine birth.10
Numerous LAMPs are encoded by the bovine ureaplasma genome, which are a mixture of cell-surface proteins and lipoproteins that interact with host cells and account for a significant amount of the cell's mass. LAMPs facilitate several key processes, including cellular adhesion and invasion, immunomodulation, inhibition or activation of apoptosis, and are the major pathogen associated molecular patterns associated with mollicute species. U. diversum undergoes internalization as quickly as one minute following its attachment to a target cell, which is soon followed by perinuclear localization. This process plays a key role in Ureaplasma's survival in immunocompetent hosts, as the intracellular environment provides the organism with both nutrients and shelter from antimicrobials that do not penetrate the host cell. This survival mechanism coincides with Ureaplasma's ability to inhibit apoptosis of infected cells.10
Ureaplasma diversum is capable of colonizing fetal lung in utero as well as newborn lung via endobronchial inoculation. As evident in this case, fetal pulmonary pathology is typically associated with lymphocytic infiltrates (i.e. BALT hyperplasia) and is often accompanied by conjunctivitis. In most cases the fetus is relatively fresh while the placenta is often retained and inflamed.10
In addition to rare cases of mycotic infections, U. diversum is one of very few etiologies associated amnionitis, which macroscopically manifests as marked thickened of the amnion. In contrast, U. diversum does not cause lesions in the chorioallantois. Optimal tissue specimens for confirmatory testing include placenta, fetal lung and fetal abomasal fluid.10
Interestingly, Ureaplasma diversum has recently been isolated from swine with pneumonia. Isolates were only obtained from sick animals, which in contrast to cattle which often have asymptomatic infections of the respiratory tract. An additional study found U. diversum was only detectable in the lung tissue of affected swine, while also discovering that coinfection with Mycoplasma spp. such as M. hyopneumonia and M. hyorhinus to be common. Additional research is needed in regard to determining if U. diversum is simply an opportunistic pathogen or is a component of the Swine Respiratory Disease complex.10
Although not seen in this case, the moderator also discussed the presence of keratinocytes within neonatal airways as an indication of fetal distress.
References:
1. Argue B, Chousalkar KK, Chenoweth PJ. Presence of Ureaplasma diversum in the Australian cattle population. Aust Vet J. 2013;91:99-101.
2. Anderson ML. Infectious causes of bovine abortion during mid- to late-gestation. Theriogenology. 2007;68:474-486.
3. Ball HJ, Neill SD, Ellis WA, et al. The isolation of Mycoplasma from bovine foetuses and their dams. Br Vet J. 1978;134:584-589.
4. Himsworth CG, Hill JE, Huang Y, Waters EH, Wobeser GA;, Destructive polyarthropathy in aborted bovine fetuses: a possible association with Ureaplasma diversum infection? Vet Pathol. 2009;46:269-272.
5. Howard CJ, Gourlay RN: 1982, Proposal for a second species within the genus Ureaplasma, Ureaplasma diversum sp. nov. Int. J. Syst. Bact. 1982;32: 446-452
6. Kreplin CM, Maitland VF: 1989, Alberta. Abortion due to Ureaplasma diversum. Can Vet J. 1989;30(5):435.
7. Miller RB, Ruhnke HL, Doig PA, Poitras BJ, Palmer NC. The effects of Ureaplasma diversum inoculated into the amniotic cavity in cows. Theriogenology. 1983;20:367-374.
8. Miller RB, Ruhnke HL, Palmer NC, Doig PA. Bovine abortion caused by Ureaplasma diversum. In: CA Kirkbride, ed. Laboratory Diagnosis of Livestock Abortion, 3rd ed., Ames, IA. Iowa State University Press; 1990: 30-36.
9. Ruhnke HL, Palmer NC, Doig PA, Miller RB. Bovine abortion and neonatal death associated with Ureaplasma diversum. Theriogenology 1984; 21:295-301.
10. Santos Junior MN, de Macêdo Neres NS, Campos GB, Bastos BL, Timenetsky J, Marques LM. A Review of Ureaplasma diversum: A Representative of the Mollicute Class Associated With Reproductive and Respiratory Disorders in Cattle. Front Vet Sci. 2021;8:572171.
11. ter Laak EA, van Dijk JE, Noordergraaf JH. Comparison of pathological signs of disease in specific-pathogen-free calves after inoculation of the respiratory tract with Ureaplasma diversum or Mycoplasma canis. J Comp Pathol. 1993;108:121-132
12. Viscardi RM. Ureaplasma species: role in diseases of prematurity. Clin Perinatol. 2010;37:393-409.
13. Viscardi RM. Ureaplasma species: role in neonatal morbidities and outcomes. Arch Dis Child Fetal Neonatal Ed. 2014;99:F87-92.
14. Watson P, Mason C, Stevenson H, et al. Laboratory diagnosis of Mycoplasma/Ureaplasma abortion in cattle. Vet Rec. 2012;170:82-84 (letter)