Wednesday Slide Conference, Conference 13, Case 4
Signalment:
12 years old and 3 months, female (neutered), Golden retriever, Canis lupus familiaris, canine
History:
The animal was surgically treated with ovariohysterectomy due to pyometra. Both ovaries and uterus were formalin-fixed and submitted for histopathological examination.
Gross Pathology:
Macroscopically, right and left ovary appeared to be increased in size. Right ovary was 3x5 cm and left ovary was 8x6 cm. In addition, both uterus and ovaries had multiple nodules and cysts, the latter containing macroscopically transparent fluid.
Laboratory Results:
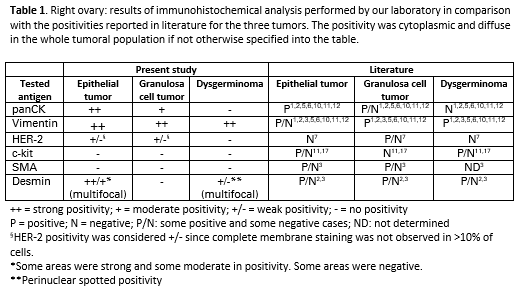
Below is a table reporting the results of the immunohistochemical analysis (Tab. 1).
Microscopic Description:
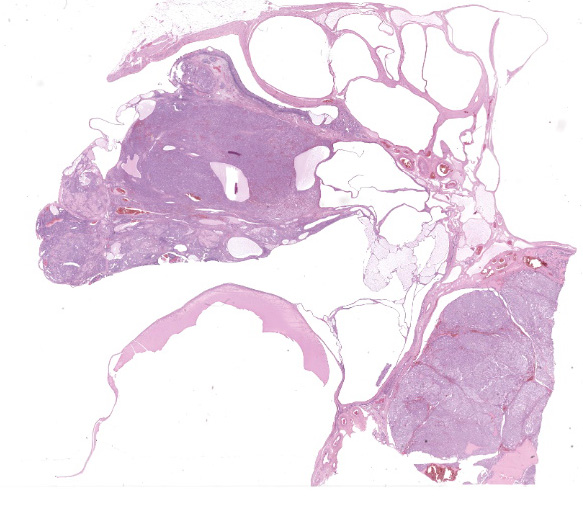
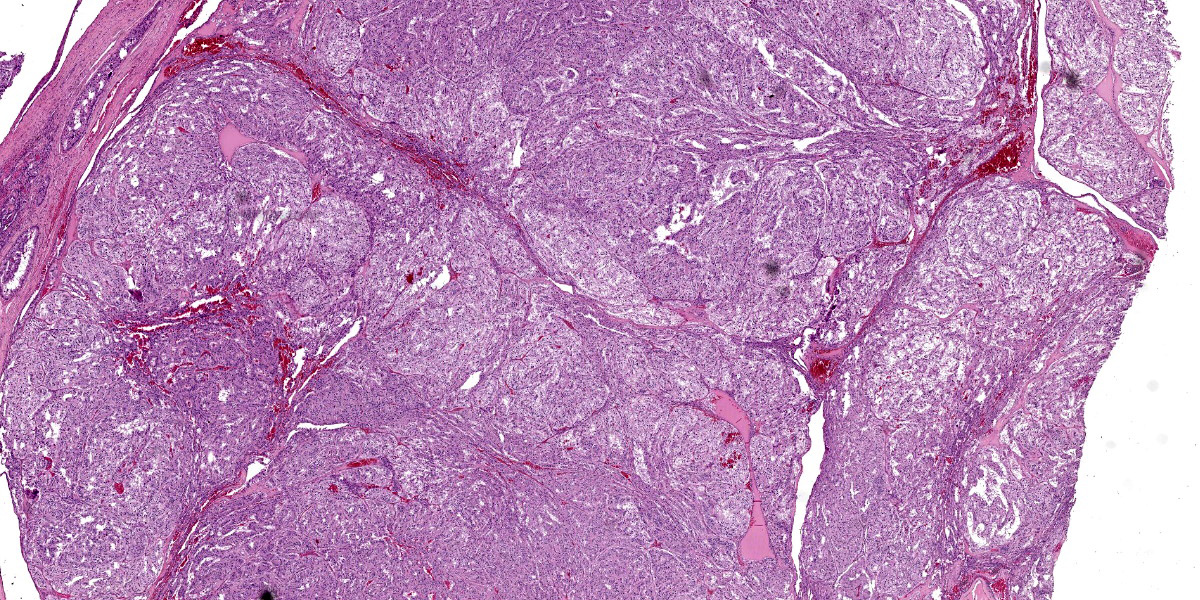
Right ovary:
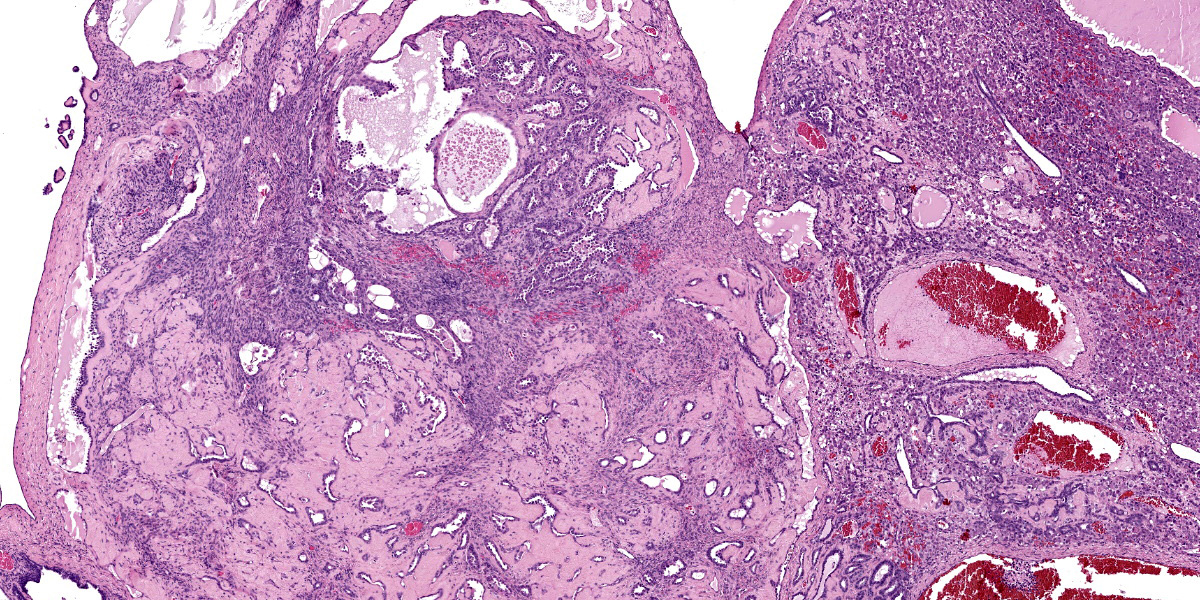
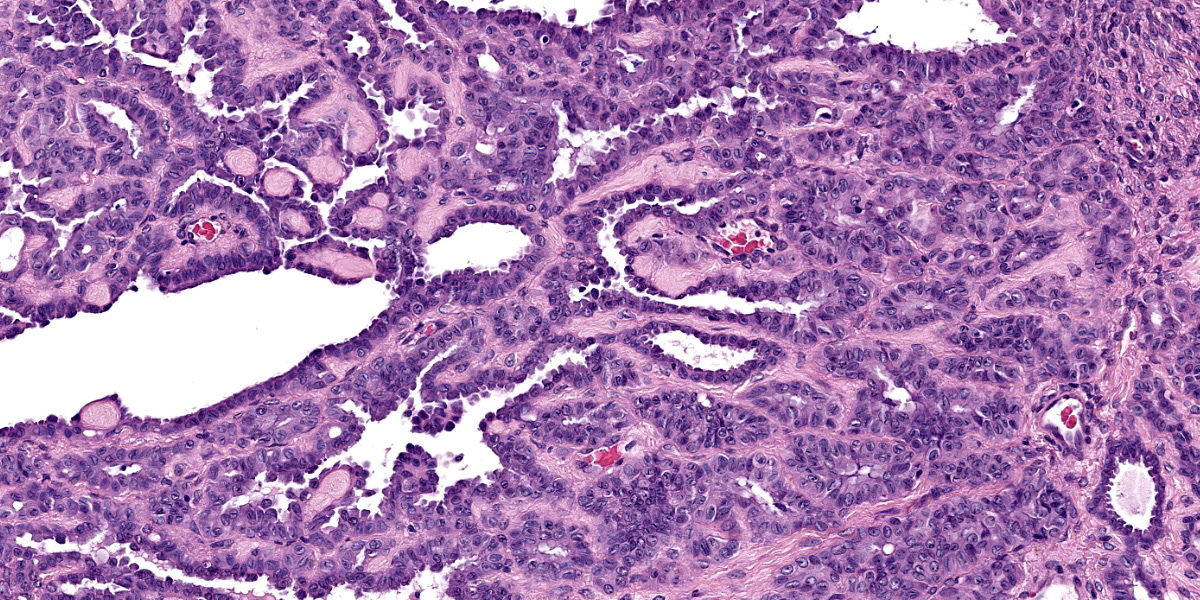
Continuous with the ovarian surface epithelium, occupying 20% of the section and replacing the normal ovarian tissue, there is a moderately cellular, not well demarcated, non-encapsulated and non-infiltrative neoplastic population. The neoplasm is characterized by endophytic and esophytic tubular and tubulo-papillary structures, associated with moderate to abundant ovarian fibrovascular stroma. Tubules are occasionally ectatic and contain a moderate amount of often homogeneously eosinophilic amorphous material.
Neoplastic cells are cuboidal to columnar with indistinct cell borders, scant eosinophilic cytoplasm and round to oval (10 um) eccentrically located nuclei with finely stippled chromatin and mainly 1 distinct nucleolus. Anisocytosis and anisokaryosis are mild. Mitoses are rare (< 1 per hpf).
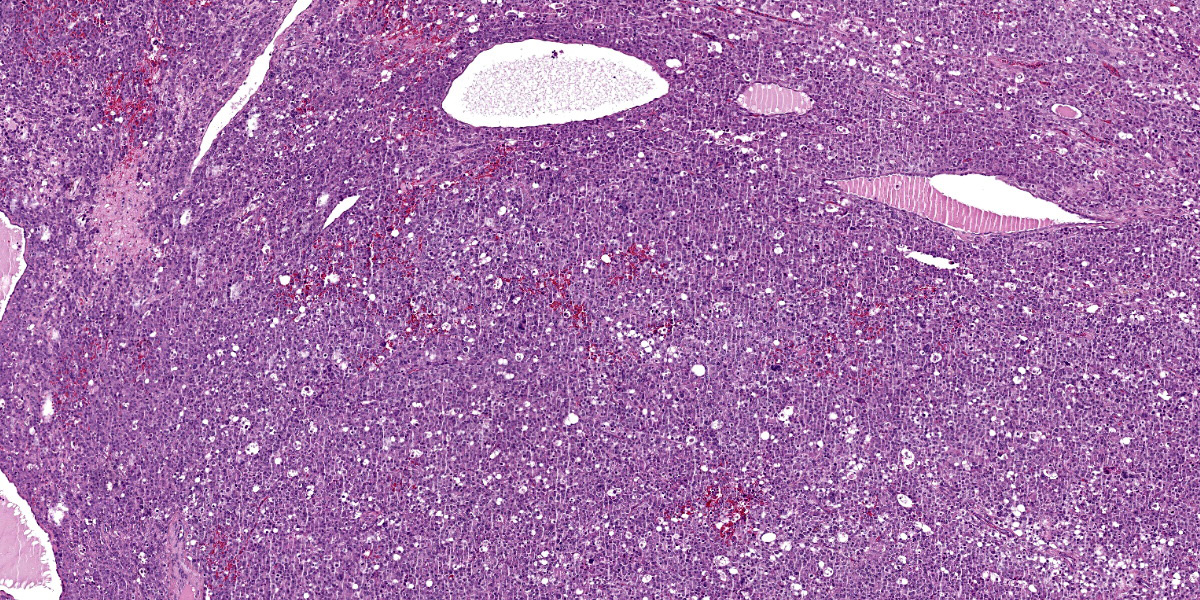
Close to this neoplasm, multifocally colliding, there is a second densely cellular, well-demarcated, non-encapsulated and infiltrative neoplasm occupying 40% of the section. The neoplasm is characterized by sheets of cells admixed with a very fine and scant fibrovascular stroma.
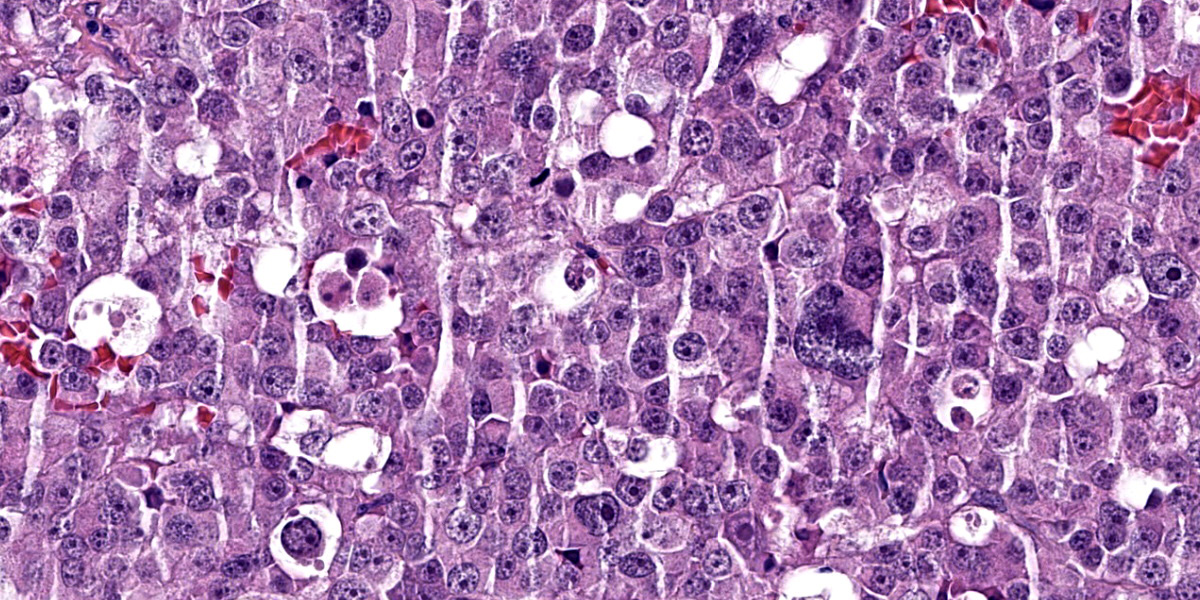
Neoplastic cells are irregularly round with distinct cell borders (up to 20 um), moderate to abundant occasionally granular slightly eosinophilic cytoplasm, occasionally showing single to multiple large empty well-defined vacuoles.
The nucleus is round to oval, rarely megalic (macrokaryosis) with finely stippled chromatin and 1 distinct nucleolus. Anisocytosis and anisokaryosis are moderate with occasional bi and multinucleation. Mitoses are on average 2-4 per high power field.
Multifocal mild hemorrhages are evident, as well as disseminated single cell necrosis/apoptosis. Entrapped in the neoplasm, there are occasional ectatic and cystic tubular structures lined by a cuboidal to flattened epithelium.
At the periphery of both the neoplasms and close or involving the rete ovarii, multiple variably-sized ovarian and para-ovarian epithelial and vascular cystic spaces occasionally containing a variable amount of slightly eosinophilic amorphous material are also observed.
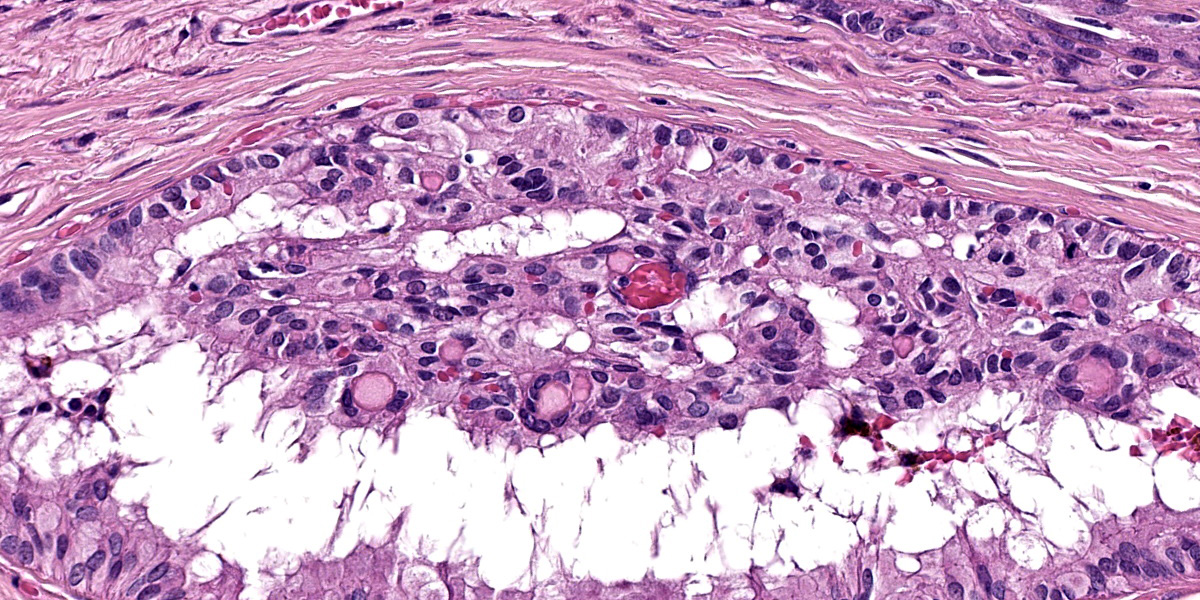
Close to the cystic portion, occupying 20% of the section there is a densely cellular, well demarcated, non-encapsulated and non-infiltrative neoplasm. The cells form mainly nest and tubular structures, the latter mainly with small lumens (microfollicular), separated by variably sized bands of scant fibrovascular stroma. Neoplastic cells are irregularly oval to elongated with indistinct cell borders, moderate eosinophilic fibrillar to vacuolized cytoplasm with small (5-7 um) hyperchromatic round to oval nuclei. Anisocytosis and anisockryosis are mild. Neoplastic cells focally surround microcavities containing intensely eosinophilic amorphous material (Call-Exner bodies). Mitoses are rare (<1 per hpf). Multifocal haemorragic lacunae are also present.
Contributor’s Morphologic Diagnosis:
Right Ovary: papillary adenoma, dysgerminoma and granulosa cell tumor associated with multiple ovarian and para-ovarian cysts.
Left ovary (not submitted): dysgerminoma
Uterus (not submitted): cystic endometrial hyperplasia and purulent endometritis
Contributor’s Comment:
This was a very unusual case of three different tumors in the same ovary of an adult female dog.
Ovarian tumors are described as a frequent condition in female dogs, but epidemiological data are incomplete. They occur more often in older animals, and usually are noted due to behavioral changes. Additionally, lactation and vaginal discharge can also be present. They also can be associated with ovarian cysts and with uterine lesions such as cystic endometrial hyperplasia and pyometra.9,16
Typically, ovarian tumors grow as single subtype either monolaterally, or less often, bilaterally with the latter being mainly represented by sex cord stromal tumors.9 Only rare cases of simultaneous neoplastic lesions of different cell of origin have been described in ovaries. To our knowledge, there is only one case report of a concomitant teratoma and granulosa cell tumor growing distinctively into the two ovaries of an English Bulldog.8

Papillary adenoma is a benign tumor of the surface epithelium mainly described in the bitch. Usually at the cut surface there are multiple small cysts which are a feature of this type of tumor.5 The neoplasm can be smooth and nodular, or when arising on the surface, give the ovary a cauliflower-like appearance. There is scant connective stroma and the papillary projections are lined by small cuboidal to cylindrical cells that may have cilia. Mitotic figures are rare.12 Papillary adenocarcinoma is the malignant counterpart of benign papillary adenomas.5 Size is an important criterion of malignancy. Tumors that extend through the opening of the ovarian bursa are likely to be malignant and spread by implantation to the peritoneal surface. The surface of an adenocarcinoma tends to be shaggy, and it is the fronds that break off and give rise to metastatic implantations. This tumor is similar in origin and appearance to its benign counterpart, and distinguishing between them can be difficult. Additional criteria for malignancy are increased mitotic activity, invasion into the ovarian stroma, and extension into the ovarian bursa.12 Based on features in this case, we favored a papillary adenoma.
Dysgerminoma is a tumor that develops from germ cells before differentiation. It has been recognized most often in the bitch, but is much less common in female dogs than the male testicular counterpart (i.e. seminoma). Dysgerminomas are malignant and may metastasize or spread locally, but early spread is not common. Histologically, there are no defined criteria to predict tendency to metastasize.12 Dysgerminomas are composed of a uniform population of large round cells with clear or light-staining amphophilic cytoplasm. The nuclei are centrally located and contain abundant granular chromatin and either one or two prominent nucleoli. Mitotic figures are often numerous, and incomplete division of tumor cells may result in multinucleated giant cells. Individual tumor cells may undergo necrosis, leaving a distinctive clear space. The stroma is usually light and may be infiltrated with lymphocytes.12
Granulosa cell tumors are composed primarily of cells that resemble the granulosa cells of the ovarian follicle. They can develop from ovarian remnants and be either benign or malignant. The tumor often contains cells of the theca interna and fibroblasts. In the bitch, they are slightly less common than epithelial tumors of the ovary.12 Steroidogenesis may be associated with granulosa cell tumors. Either estrogens or androgens can be produced, though not all granulosa cell tumors are hormonally active. Inhibin is regularly produced by granulosa cell tumors in the mare and is thought to be the cause of atrophy of the contralateral ovary.12 Only rarely do granulosa cell tumors metastasize in cows or bitches, and such reports are even rarer in mares.12 Neoplastic granulosa cells have spherical-to-oval, hyperchromatic nuclei, distinct nucleoli, and scant eosinophilic cytoplasm. The common patterns are follicular (microfollicular and macrofollicular), insular, trabecular, and diffuse. Often a combination of patterns exists in a single tumor. Call-Exner bodies, which are distinctive microcavities that contain watery or hyaline eosinophilic material and occasionally pyknotic nuclei are surrounded by granulosa cells in a rosette arrangement. These are seen most often in the microfollicular pattern. Some granulosa cell tumors, particularly those in the bitch, develop a tubular pattern similar to that of the Sertoli cell tumor of the testis, and like Sertoli cell tumors some of these granulosa cell tumors induce a dense fibrous stroma. Theca cells may also be present in granulosa cell tumors. Either or both cell types may be luteinized.12
Expression markers of different normal ovarian cell type (Table 2) can be helpful to characterize tumors of different origin particularly when less differentiated as also reported in Table 1. The tumors reported in this case presented a IHC phenotype resembling what reported into the literature for the studied markers (Table 1).
In addition to the markers performed by our laboratory, CK7, PLAP and inhibin could also be performed to further confirm the tissue origin of the identified neoplasms.1,2,5,6,10,11,12
Contributing Institution:
Department of Comparative Biomedicine and Food Science (Università of Padua)
Viale dell’Università, 16 (35020), Legnaro (PD), Italy
https://www.bca.unipd.it/
JPC Diagnosis:
1. Ovary: Dysgerminoma.
2. Ovary: Papillary adenoma.
3. Ovary: Granulosa cell tumor.
4. Ovary: Cysts, multiple.
JPC Comment:
The final case of this conference features four separate entities to describe. Conference participants noted one or more neoplasms and cysts, though only a select few were tenacious enough to identify all 3 neoplastic cell populations. This is a unique slide that is a microcosm of ovarian neoplasia – it is a fine companion to the analogous testicular tumor case we reviewed in WSC 24-25 Conference 10 (Case 2 – also from a dog).
We agree with the contributor that multiple different ovarian neoplasms is not a common finding in the dog. Ovarian neoplasia itself is relatively rare in dogs, representing approximately 1-2% of all neoplasms.14,15 Arriving at 3 separate neoplasms in a single ovary likely represents the confluence of multiple rare outcomes. That the contralateral ovary in this case (not included with this slide) also had a dysgerminoma could reflect either metastasis or a separate neoplasm arising spontaneously – the submission materials leave this aspect of the case open.
Identification of a granulosa cell tumor was straightforward in this case with recognition of Call-Exner bodies and fibrous tissue septa being helpful correlates. The intersection of the dysgerminoma and the adenoma is intriguing, but the two neoplasms are easily distinguished from each other. .We considered the possibility of a ovarian carcinoma in this case, however, definitive areas of stromal invasion are not evident in these sections.
Lastly, we agreed with the contributor that there are multiple cysts present in this case, though we did not attempt to further classify each one, especially in light of the three distinct neoplasms in the surrounding tissue. Cystic structures arising from the ovary include cysts of the rete ovarii, subsurface epithelial structures, and follicle. Paraovarian cysts are located in tissue adjacent to the ovary and include remnants of the mesonephric duct, paramesonephric duct, and mesonephric tubule.13 Features such as the presence of ciliated epithelium, smooth muscle in the cyst wall, and a basement membrane can be used to distinguish these diagnoses.13 Location (if known) is also helpful discriminator.
References:
- Auersperg N, Wong AST, Choi K-C, Kang SK, Leung PCK. Ovarian Surface Epithelium: Biology, Endocrinology, and Pathology. 2001;22(2).
- Akihara Y, Shimoyama Y, Kawasako K, et al. Immunohistochemical Evaluation of Canine Ovarian Tumors. J Vet Med Sci. 2007;69(7):703–708.
- Czernobilsky B, Shezen E, Lifschitz-Mercer B, et al. Alpha smooth muscle actin (alpha-SM actin) in normal human ovaries, in ovarian stromal hyperplasia and in ovarian neoplasms. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57(1):55-61.
- Dolenšek T, Knific T, Ramírez GA, Erles K, Mallon HE, Priestnall SL, Suárez-Bonnet A. Canine ovarian epithelial tumours: histopathological and immunohistochemical evaluation with proposed histopathological classification system. J Comp Pathol. 2024 Jul;212:42-50.
- Kennedy PC, Cullen JM, Edwards JF, Goldschmidt MH, Larsen S, Munson L, Nielsen. Histological classification of tumors of the genital system of domestic animals. WHO, Washington, DC: Armed Forces Institute of Pathology; 1998.
- MacLachlan NJ, Kennedy PC: Tumors of the genial system. In Meuten DJ: Tumors in Domestic Animals. 4th ed. Ames, IO: Iowa State Press;2002:547-573.
- Matos ACHDS, Consalter A, Santos Batista BP, Fonseca ABM, Ferreira AMR, Leite JDS. Immunohistochemical expression of HER ? 2 and Ki ? 67 in granulosa cell tumor in bitches. Reprod Dom Anim. 2021;56(4):667–672
- Oviedo-Peñata CA, Hincapie L, Riaño-Benavides C, Maldonado-Estrada JG. Concomitant Presence of Ovarian Tumors (Teratoma and Granulosa Cell Tumor), and Pyometra in an English Bulldog Female Dog: A Case Report. Front Vet Sci. 2020;6:500.
- Patnaik AK, Greenlee PG. Canine Ovarian Neoplasms: A Clinicopathologic Study of 71 Cases, Including Histology of 12 Granulosa Cell Tumors. Vet Pathol. 1987;24(6):509–514.
- Riccardi E, Greco V, Verganti S, Finazzi M. Immunohistochemical Diagnosis of Canine Ovarian Epithelial and Granulosa Cell Tumors. J Vet Diagn Invest. 2007;19(4):431–435
- Rosa RB, Bianchi MV, Ribeiro PR, et al. Comparison of immunohistochemical profiles of ovarian germ cells in dysgerminomas of a captive maned wolf and domestic dogs. J Ver Diagn Invest. 2021;33(4):772–776.
- Schlafer DH, Miller RB: Female genital system. In: Maxie MG, ed.. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 5th ed. Philadelphia, PA: Elsevier Limited; 2007, vol 3: 450-456
- Schlafer DH, Foster RA. Female Genital System. In: Maxie MG, ed. Jubb, Kennedy & Palmer's Pathology of Domestic Animals. Vol 3. 6th ed. St. Louis, MO: Elsevier; 2016:358-464.
- Sforna M, Brachelente C, Lepri E, Mechelli L. Canine ovarian tumours: a retrospective study of 49 cases. Vet Res Commun. 2003;27:359–361.
- Troisi A, Orlandi R, Vallesi E, Pastore S, Sforna M, Quartuccio M, Zappone V, Cristarella S, Polisca A. Clinical and ultrasonographic findings of ovarian tumours in bitches: A retrospective study. Theriogenology. 2023 Oct 15;210:227-233.
- Walter B, Coelfen A, Jäger K, Reese S, Meyer?Lindenberg A, Aupperle?Lellbach H. Anti?Muellerian hormone concentration in bitches with histopathologically diagnosed ovarian tumours and cysts. Reprod Domestic Animals. 2018;53(3):784–792.
- Yi C, Li L, Chen K, Lin S, Liu X. Expression of c-Kit and PDGFRα in epithelial ovarian tumors and tumor stroma. Oncol Lett. 2012 Feb;3(2):369-372.