Signalment:
Gross Description:
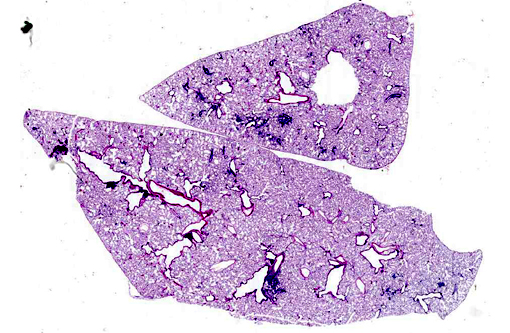
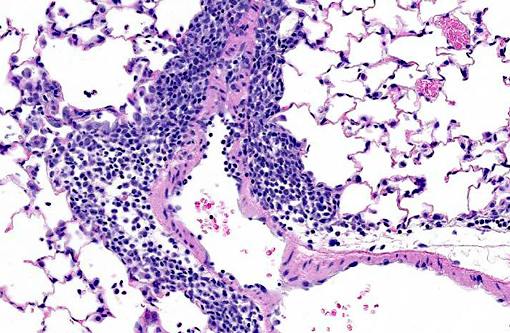
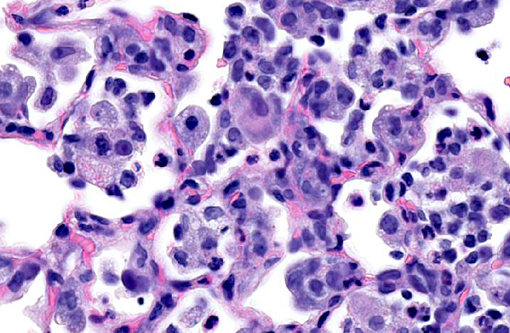
Histopathologic Description:
09-1215 - H10-0126A
09-1220 H10-0131A
Lung: Multifocally, there are moderate amounts of perivascular, peri-bronchiolar, interstitial and alveolar hypercellularity. The majority of cells are pleomorphic lymphocytes and large foamy macrophages with fewer neutrophils and occasional plasma cells and eosinophils (mixed inflammatory cell interstitial pneumonia). In some slides the pleura is multifocally mildly expanded by a similar cellular infiltrate (pleuritis). Multifocally, the alveoli are filled with variable numbers of large foamy alveolar macrophages, occasional multinucleate giant cells, moderate amounts of cellular debris and are occasionally lined by plump cuboidal epithelium (mild type II pneumocyte hyperplasia). The perivascular lymphatics are occasionally moderately ectatic (perivascular oedema).
Morphologic Diagnosis:
Lab Results:
1. Aerobic and anaerobic culture of lung tissue did not culture any bacteria or fungi.
2. Surveillance of sentinel rats during this investigation was negative for pneumonia virus of mice, Theilers encephalomyelitis virus (GD VII), Hantaan virus (Korean hemorrhagic fever), lymphocytic choriomeningitis virus, Sendai virus, reovirus-3, sialodacryoadenitis (rat coronavirus), rat parvoviruses (rat virus, rat parvovirus-1, Toolan H1 virus), Corynebacterium kutscheri, Bordatella bronchiseptica, Pasteurella multocida, Streptococcus pneumonia, Streptococcus moniliformis, Staphylococcus aureus, Mycoplasma pulmonis, CAR bacillus, Clostridium piliforme, Salmonella enteriditis, Helicobacter sp., nematodes, cestodes, intestinal protozoa (pathogenic species), Encephalitozoon cuniculi and arthropods.Â
3. PCR testing for Pneumocystis carinii was positive in lung tissue harvested from both rats at post mortem.
Condition:
Contributor Comment:
Further research undertaken in an attempt to isolate and transmit an infectious cause for the lung lesions led to the description of a novel enveloped virus and the disease was given the working name rat respiratory virus (RRV).(5,6) However, despite these early findings, other attempts at virus isolation from infected lungs were unsuccessful and the etiology of the pulmonary inflammatory lesions also known as RRV was undetermined and histopathology remained the only method of diagnosis (Albers, Simon et al. 2009).Â
In 2011 and 2012, two independent research groups, released publications containing convincing evidence that the fungal agent Pneumocystis carinii was the cause of the distinctive lung lesions known as RRV. Techniques used to demonstrate the correlation between the presence of P. carinii and the presence of the characteristic lung lesions included PCR, serology and experimental infection studies.(Livingston, Besch-Williford et al. 2011, Henderson, Dole et al. 2012). The gross and histopathological changes in the rat lungs submitted, combined with the negative microbial culture and serological testing results and positive PCR results for Pneumocystis carinii, led to the diagnosis of Pneumocystis pneumonia in these rats.
JPC Diagnosis:
Conference Comment:
P. carinii typically requires immune suppression for infection to cause clinical disease. In humans the organism is hypothesized to be continually present within the lungs from the first few years of life, often in the absence of overt pathology.(8) In certain rat colonies, P. carinii is apparently common and has been documented in the lungs of clinically healthy, immunocompetent rats; research suggests neonatal rats acquire it within hours after birth.(4) A survey of research institutions revealed 6% of rats had the characteristic perivascular lymphocyte accumulation associated with that described in the 1990s for RRV.(4) These lesions were reproduced experimentally in immunocompetent rats by inoculation of P. carinii and its transmissible potential was documented in independent studies.(3,4) Further, organism identification by PCR was highly correlated with lung pathology, and the organism was never identified in cases lacking the characteristic lung lesions, allowing characteristic microscopic changes to be an accurate predictor of P. carinii infection.(3) Despite the evidence, definitive causation can still not be assigned as the inoculum utilized in both studies was derived from infected rat lungs, thus the possibility of a second or primary agent remains, though that possibility is argued as highly improbable.4 Regardless, both experimental and natural infections spontaneously resolve with time.(1,3)
References:
1. Albers TM, Simon MA, Clifford CB. Histopathology of naturally transmitted "rat respiratory virus": progression of lesions and proposed diagnostic criteria. Vet Pathol. 2009;46(5):992-999.Â
2. Elwell MR, Mahler JF, Rao GN. Have you seen this? Inflammatory lesions in the lungs of rats. Toxicol Pathol 1997;25:529-531.
3. Henderson KS, Dole V, Parker NJ, Momtsios P, Banu L, Brouillette R, Simon MA, Albers TM, Pritchett-Corning KR, Clifford CB, Shek WR. Pneumocystis carinii causes a distinctive interstitial pneumonia in immunocompetent laboratory rats that had been attributed to "rat respiratory virus. Vet Pathol. 2012;49(3):440-52.Â
4. Livingston RS, Besch-Williford CL, Myles MH, Franklin CL, Crim MJ, Riley LK. Pneumocystis carinii infection causes lung lesions historically attributed to rat respiratory virus Comp Med. 2011;61(1):45-59.
5. Riley L, Purdy G, Dodds J, et al Idiopathic lung lesions in rats: search for a etiologic agent Contemp Top Lab Anim Sci. 1997;36:46.
6. Riley L, Simmons J, Purdy G, et al. Research Update: idiopathic lung lesions in rats. ACLAD News. 1999;20:9-11
7. Slaoui M, Dreef HC, van Esch. Inflammatory lesions in the lungs of Wistar rats. Toxicol Pathol 1998;26:712-713, discussion 714.
8. Swain SD, Meissner N, Han S, Harmsen A. Pneumocystis infection in an immunocompetent host can promote collateral sensitization to respiratory antigens. Infect Immun. 2011;79(5):1905-1914.