Signalment:
Histopathologic Description:
Morphologic Diagnosis:
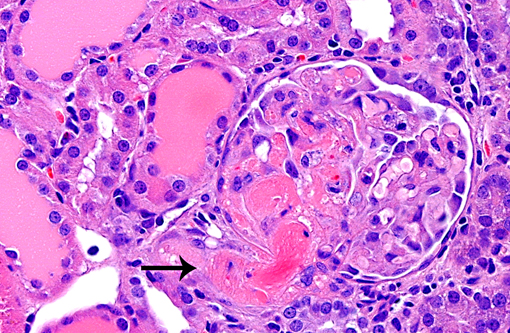
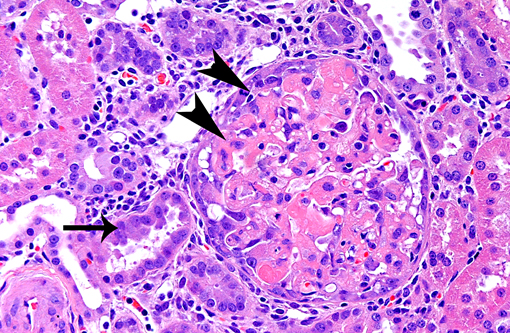
1. Glomerulonephropathy, multifocal, chronic with glomerular hyalinosis and sclerosis and tubular degeneration/ regeneration, atrophy, ectasia and proteinuria
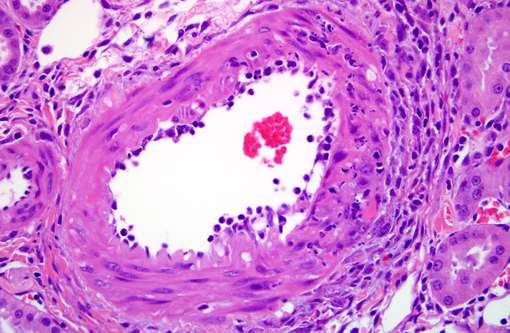
2. Arteriolopathy, proliferative, chronic, multifocal with medial degeneration and fibrinoid necrosis
Condition:
Contributor Comment:
In experimental medicine, rats are the most popular hypertensive model and the spontaneous hypertensive rat (SHR) is the most common model utilized.(9) Impaired endothelium dependent relaxation, cardiac hypertrophy and/or heart failure, cerebral hemorrhage, nephropathy and/or renal failure are features of most models of rat hypertension, mimicking the human spectrum of disease. Hypertension in rats is defined as sustained systolic blood pressure of greater than 150 mmHg.(2) The submitted case illustrates microscopic features that developed after 14 days of hypertension induced by high salt diet and angiotensin II infusion. Table 1 compares selected systemic hypertensive rat models.
| Rat Model | Hyper-tension Etiology | Hypertension Mechanism of Action | Age of Onset | Blood Pressure Elevation (Systolic) | Comments |
| Stroke-prone Spontaneously Hypertensive Rat (SHRSP)(2,9) | Primary, genetic | Renally related -- Transplanting a kidney from SHR to a normotensive Wistar rat increases blood pressure in the recipient | Begins at 6-7 weeks of age | 200 mmHg | 80% die from stroke |
| Spontaneously Hypertensive Rat (SHR)(2,9) | Primary, genetic | Renally related -- Transplanting a kidney from SHR to a normotensive Wistar rat increases blood pressure in the recipient | Begins at 5-6 weeks of age, maximal by 12 weeks | 180-200 mmHg | 30% develop heart failure at 4-5 months of age |
| Dahl Salt Sensitive Rat(9) | Primary, genetic and dietary | High dietary sodium content increases circulating sodium causing osmotic pull into vasculature, elevating pressure on vessel walls | 4-6 weeks of age | Increased even on normal diet; Steeply elevated on high salt diet | 30% develop heart failure at 18 months of age |
| Transgenic TGR(mRen2)27 Rat(4,9) | Primary, genetic | Overexpression of the mouse Ren-2 gene (increased renin activity) | Begins at 5 weeks and maximal by 10 weeks of age | Heterozygous: 240 mmHg; Homozygous: 300 mmHg (high mortality) | Mortality from heart failure at 10 weeks of age |
| Double Transgenic (dTG) Rat(11) | Primary, genetic | Expresses both human renin and angiotensinogen genes | Early | 200-220 mmHg | 50% mortality by 7-8 weeks of age |
| Deoxycorticosterone Acetate (DOCA) + High Salt Diet(2,7, 9) | Secondary, endocrine/ dietary | Salt-dependent, mineralocorticoid (DOCA)-induced reabsorption of salt and water resulting in increased blood volume Also increased secretion of vasopressin = vasoconstriction + water retention | Hyper-tension begins 1-2 weeks after start of DOCA and high salt diet | 200 mmHg | Mortality from brain, vascular, and renal lesions after 4-8 weeks of treatment |
| Ang II Infusion and High Salt Diet(10) | Secondary, endocrine | Daily infusion of angiotensin II via osmotic pump and high salt diet | Begins 1 day post infusion | 130-200 mmHg | Mortality from renal glomerulosclerosis and vascular necrosis |
| Goldblatt Method: Two-Kidney One-Clip(2,9) | Secondary, renal | Clip on one renal artery increases circulating renin and angiotensin II | Develops 6 weeks post-surgery | 160 - 190 mmHg | Mortality from renal failure and cardiovascular complications |
| Goldblatt Method: Two-Kidney Two-Clip(2) | Secondary, renal | Partial occlusion of both renal arteries (two stage surgery) increases circulating renin and aldosterone | Develops 4 weeks post-surgery | 160 - 190 mmHg | |
| Godlblatt Method: One-Kidney One Clip(2) | Secondary, renal | Uninephrectomy and clip on renal artery of remaining kidney with rapid salt and water retention; Plasma renin activity is normal | Within hours of surgery | 160 - 190 mmHg | |
| Renal Remnant(12) | Secondary, renal | Uninephrectomy and segmental 2/3 ablation of remaining kidney to 5/6 total renal mass | Within hours of surgery | 170-190 mmHg | Mortality from renal failure with cardiovascular complications |
Angiotensin II has a wide array of biological effects including (from Kobori et. al(3)):
1. Arteriolar vasoconstriction (in the glomerulus, efferent > afferent)
2. Stimulation of aldosterone secretion (zona glomerulosa of the adrenal cortex)
-Ç-ó Acts on distal convoluted tubules and collecting ducts → reabsorb sodium and water from urine (exchange for potassium which is excreted in urine) → ↑blood volume → ↑blood pressure
3. Secretion of anti-diuretic hormone from the pituitary
a. Vasoconstriction
b. Reabsorption of water in the kidneys
c. Stimulation of thirst and salt appetite
4. Regulation of sodium transport by renal and intestinal epithelium
-Ç-ó In the kidney, stimulation of Na+/H+ exchange on proximal tubules, thick ascending limb of the loop of Henle, and collecting ducts → increased sodium resorption
5. Hypertrophy of renal tubular epithelium
6. Release of prostaglandins → counteracts renal vasoconstriction
7. Reduced renal medullary blood flow
8. Increases tubuloglomerular feedback sensitivity → lower tubular perfusion (prevents excessive rise in glomerular filtration rate)
9. Other actions
a. Enhanced cardiomyocyte growth and contractility
b. Stimulation of release of catecholamines (norepinephrine from adrenal medulla)
c. Increases sympathetic nervous system activity
JPC Diagnosis:
1. Kidney, arterioles: Arteriopathy, proliferative and necrotizing.
2. Glomerulosclerosis, multifocal, moderate, with tubular degeneration, regeneration, and protein casts.
Conference Comment:
The contributor has provided an excellent comparative review of hypertensive nephropathy, a condition that is morphological similar in most affected species.
References:
2 Doggrell SA, Brown L: Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res 39: 89-105, 1998
3 Kobori H, Nangaku M, Navar LG, Nishiyama A: The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251-287, 2007
4 Langheinrich M, Lee MA, Bohm M, Pinto YM, Ganten D, Paul M: The hypertensive Ren-2 transgenic rat TGR (mREN2)27 in hypertension research. Characteristics and functional aspects.Am J Hypertens 9: 506-512, 1996
5 Marcantoni C, Fogo AB: A perspective on arterionephrosclerosis: from pathology to potential pathogenesis. J Nephrol 20: 518-524, 2007
6 Navar LG: The kidney in blood pressure regulation and development of hypertension. Med Clin North Am 81: 1165-1198, 1997
7 Park CG, Leenen FH: Effects of centrally administered losartan on deoxycorticosterone-salt hypertension rats. J Korean Med Sci 16: 553-557, 2001
8 Percy DH, Barthold SW. Pathology of Laboratory Rodents and Rabbits. 3rd ed., Ames, Iowa:Blackwell Publishing; 2007: 161-4.
9 Pinto YM, Paul M, Ganten D: Lessons from rat models of hypertension: from Goldblatt to genetic engineering. Cardiovasc Res 39: 77-88, 1998
10 Rugale C, Delbosc S, Cristol JP, Mimran A, Jover B: Sodium restriction prevents cardiac hypertrophy and oxidative stress in angiotensin II hypertension. Am J Physiol Heart Circ Physiol 284: H1744-1750, 2003
11 St-Jacques R, Toulmond S, Auger A, Binkert C, Cromlish W, Fischli W, Harris J, Hess P, Lan J, Liu S, Riendeau D, Steiner B, Percival MD: Characterization of a stable, hypertensive rat model suitable for the consecutive evaluation of human renin inhibitors. J Renin Angiotensin Aldosterone Syst, 2011
12 Sviglerova J, Kuncova J, Nalos L, Tonar Z, Rajdl D, Stengl M: Cardiovascular parameters in rat model of chronic renal failure induced by subtotal nephrectomy. Physiol Res 59 Suppl 1: S81-88, 2010